
Wnt7a and miR-370-3p: new contributors to bladder cancer invasion
In eukaryotes, microRNAs (miRNAs) are central regulators of gene expression. These small noncoding RNAs are about 19–24 nucleotides long (1). miRNAs can silence gene expression by binding to a complementary sequence in the 3'-UTR of the target gene, which then triggers mRNA degradation. miRNAs can inhibit gene expression by also blocking translation. After an miRNA is processed into its mature form, it is loaded onto the RNA-induced silencing complex (RISC). This complex guides the miRNA to its target sequence which can reside in more than 60% of protein-coding genes. In the context of cancer, some miRNAs are categorized as oncomiRs, i.e., miRNAs that play a role in tumor initiation, metastasis, immune evasion, and angiogenesis (2). On the opposite end of the spectrum, tumor suppressor miRNAs can inhibit cancerous phenotypes by silencing oncogenes. An example of an oncomiR is miR-221. This miRNA silences anti-cancer genes such as PUMA, p27, and Beclin-1 in various cancer types including breast, lung, and prostate (2).
Bladder cancer is one of the most common cancers of urological system and is heavily associated with environmental factors such as smoking and chronic urinary tract infections (3). Patients diagnosed with muscle invasive bladder cancer have the poorest prognosis with 10-year survival rates of about 50%. Patients with non-muscle-invasive bladder cancer have better prognosis, but many patients eventually relapse and progress to muscle invasive bladder cancer (4,5). These observations highlight the importance of identifying therapeutics for treating muscle-invasive malignancy. Researchers are actively pursuing the identification of drug targets, since surgery currently remains the only curative treatment of non-invasive bladder cancer. Unfortunately, bladder cancer has also been shown to be chemoresistant. Many preclinical models have been developed to help define the underlying mechanisms and fill gaps in our understanding of this disease (4,5). p53/RB, PI3K/mTOR, FGFR3 and RAS-MAPK signaling pathways have been implicated in both types of bladder cancer. However, due to the heavy influence of environmental factors, epigenetic mechanisms such as histone modifications have also been implicated in disease progression (3). Studies using microarrays and public databases identified several miRNAs that are dysregulated in bladder cancer models and in patients (6). One study identified miR-133a-5p as a miRNA downregulated in bladder cancer tissues, which could be clinically relevant. However, no research has been reported on the role this miRNA may play in bladder cancer pathogenesis.
A recent study by Huang et al. found miR-370-3p downregulated in bladder cancer patient samples (7). To date, the role of this miRNA has not been explored in bladder cancer by other researchers. miR-370-3p was previously shown to be downregulated in glioblastoma tissues as compared to normal tissues (8). When expression was restored, glioma cells were sensitized to temozolomide, a common chemotherapeutic used for the treatment of glioblastoma. Overexpression of miR-370-3p in GBM also inhibited cellular proliferation and induced cell cycle arrest (9). This study also found that the canonical Wnt signaling pathway was inhibited through the direct silencing of β-catenin by miR-370-3p.
The Wnt signaling pathway is a highly conserved pathway that plays a seminal role in multiple biological processes (10). The Wnt cascade is dependent on the stability of β-catenin. In the absence of a Wnt ligand interacting with the Frizzled/LRP receptors, β-catenin is bound to a complex including Axin, APC, WTX, CK1, and GSK3β. In this context, β-catenin is rapidly phosphorylated, ubiquitinated, and then degraded. In the presence of Wnt ligands, Axin relocates to the LRP tail of the receptor and β-catenin can no longer be sequestered and degraded by the complex. β-catenin is then translocated to the nucleus where it can bind to and activate TCF transcription factors. Subsequently, this interaction induces expression of Wnt target genes (depicted in Figure 1) The Wnt pathway is susceptible to regulation by miRNAs at almost any position in the cascade (11). miR-34 has been reported to target Wnt ligand, β-catenin, and LRP6. β-catenin is also reported to be targeted by miR-370-3p (9) and miR-34 (11). In brain, miR-135a2 can target GSK3β and miR-310 has been shown to target TCF (11).
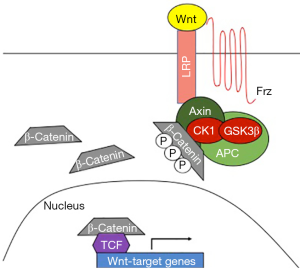
The combination of regulation by miRNAs and direct gene mutations in Wnt signaling play an important role in cancer metastasis. Potentially, one of the most important consequences of aberrant Wnt signaling is development of cancer stem cells (CSCs) (12). CSCs can initiate relapse or lead to metastasis in distant organs. Wnt signaling has a direct role in metastasis by activating epithelial-to-mesenchymal transition (EMT). Wnt target genes such as matrix metalloproteinases, fibronectin, and S100A4 also have important roles in invasion and metastasis. MMP10 is a specific matrix metalloproteinase that is activated by Wnt signaling (13). Not only can it participate in the maintenance of stemness in CSCs, but it influences tumor initiation, and metastasis (14). In esophageal squamous cell carcinoma, MMP10 is associated with poor patient survival. This is likely due to the ability of MMP10 to degrade the extracellular matrix thereby enabling tumor cell migration (15). In a lung cancer model, MMP10 is overexpressed in metastatic lesions (14).
The activity of the Wnt signaling pathway relies on the interaction of a Wnt ligand with the Frizzled/LRP receptor (10). As such, mutations in Wnt ligands can cause activation or inactivation of this important signaling pathway. Wnt7a is a member of the Wnt family that appears to have a controversial role in cancer. It is downregulated in non-small cell lung cancer (16) as well as cervical cancer (17). With the loss of expression, these cancers display increased cell migration, proliferation and tumorigenesis. However, its tumor suppressive properties appear to be independent of β-catenin signaling (16). The study by Huang et al. (7) delves into the potential of Wnt7a as a promoter of metastasis in the context of bladder cancer.
Bladder cancer does not escape the impact of aberrant Wnt signaling. A recent study found 20 single nucleotide mutations in the Wnt signaling pathway associated with bladder cancer risk (18). Another study found epigenetic silencing of Wnt inhibitory factor 1 (Wif-1) could upregulate Wnt signaling in bladder cancer tissues (19). This led to the upregulation of c-myc and increased cell growth. Cullin 4B (CUL4B) has been found to play a role in EMT of bladder cancer tissues through the upregulation of Wnt signaling which led to an increase in migration and invasion (20). MMP10 is also reported to be upregulated in bladder cancer cells due to an early mutation in FGFR3 which has a well-studied role in bladder cancer (21). Accordingly, scrutinizing aberrant Wnt signaling in the context of bladder cancer may uncover useful insights into potential targets for developing future therapeutics.
The recent study by Huang et al. set out to explore the mechanisms behind urinary bladder cancer (UBC) metastasis (7). UBC cell lines with high and low invasive capabilities were generated to reveal differentially regulated genes. Through mass spectroscopy analyses, Wnt7a, MMP10, MMP1, and S100A8 were identified as up-regulated proteins in the highly invasive cell line. As discussed earlier, aberrant Wnt signaling can contribute to metastasis. The silencing of Wnt7a in UBC cell lines inhibited invasion, EMT-associated proteins, and MMP1 and MMP10. When Wnt7a was upregulated, the opposite effects were evident. The cells acquired a more invasive phenotype as EMT-associated proteins and matrix metalloproteinases were now upregulated. These results combined with the finding that Wnt7a is upregulated in UBC patient samples supports the hypothesis that Wnt7a has a putative role in metastasis of UBC. The authors found that Wnt7a activated the canonical signaling pathway and a downstream effect is the induction of MMP10 expression through the activation of its promoter. Finally, to determine how Wnt7a is dysregulated in this model the authors focused on miRNAs. A miRanda algorithm was used and this approach identified 12 putative miRNAs targeting Wnt7a. miR-370-3p was one miRNA downregulated in UBC patient samples with lymph node invasions, but not in samples without lymph node invasions. The expression levels of miR-370-3p was evaluated in 5637 HMI (high invasive) and 5637 NMI (low invasive) cells. Expression levels of miR-370-3p was about 50% in 5637 HMI cells than that in 5637 NMI cells, suggesting that miR-370 might be relevant in UBC invasion and metastasis, but not in tumorigenesis. Overexpression of miR-370-3p downregulated Wnt7a and inhibited the invasive phenotype.
Overall, this study provides much needed insight into molecular mechanism of UBC metastasis beginning with the loss of miR-370-3p expression. The loss of this miRNA causes the upregulation of Wnt7a and consequently the activation of the Wnt signaling pathway, as well as upregulation of MMP10. The degradation of the extracellular matrix combined with active Wnt signaling can allow UBC cells to invade other tissues. Once UBC metastasizes, much like any cancer, the mortality rate for patients drastically increases. Targeting Wnt7a may seem controversial based on its variable role in other cancers (16,17). However, in the case of UBC, it is shown by this study Wnt7a may have potential as a clinically relevant diagnostic marker and drug target. Exploring miR-370-3p and Wnt7a and MMP10 protein dysregulation has provided a clearer picture of how UBC can metastasize and which genes are vital to this phenotype.
Acknowledgments
Funding: Research support from the National Institutes of Health and the National Cancer Institute (NIH/NCI): R01 CA168517 (Maurizio Pellecchia and Paul B. Fisher), and the National Foundation for Cancer Research (NFCR): Fellow grant to Paul B. Fisher is acknowledged.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Executive Editor-in-Chief Dr. Hualin Sun (Jiangsu Key Laboratory of Neuroregeneration, Nantong University, Nantong, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/biotarget.2018.08.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pradhan AK, Emdad L, Das SK, et al. The Enigma of MiRNA Regulation in Cancer. Adv Cancer Res 2017;135:25-52. [Crossref] [PubMed]
- Pradhan AK, Talukdar S, Bhoopathi P, et al. mda-7/IL- 24 mediates cancer cell-specific death via regulation of miR-221 and the beclin-1 axis. Cancer Res 2017;77:949-59. [Crossref] [PubMed]
- Li HT, Duymich CE, Weisenberger DJ, et al. Genetic and Epigenetic Alterations in Bladder Cancer. Int Neurourol J 2016;20:S84-94. [Crossref] [PubMed]
- DeGraff DJ, Robinson VL, Shah JB, et al. Current preclinical models for the advancement of translational bladder cancer research. Mol Cancer Ther 2013;12:121-30. [Crossref] [PubMed]
- Talukdar S, Emdad L, Das SK, et al. Noninvasive approaches for detection and monitoring bladder cancer. Expert Rev Anticancer Ther 2015;15:283-94. [Crossref] [PubMed]
- Wei Y, He R, Wu Y, et al. Comprehensive investigation of aberrant microRNA profiling in bladder cancer tissues. Tumour Biol 2016;37:12555-69. [Crossref] [PubMed]
- Huang X, Zhu H, Gao Z, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by MIR-370-3p. J Biol Chem 2018;293:6693-706. [Crossref] [PubMed]
- Gao YT, Chen XB, Liu HL. Up-regulation of miR-370-3p restores glioblastoma multiforme sensitivity to temozolomide by influencing MGMT expression. Sci Rep 2016;6:32972. [Crossref] [PubMed]
- Peng Z, Wu T, Li Y, et al. MicroRNA-370-3p inhibits human glioma cell proliferation and induces cell cycle arrest by directly targeting β-catenin. Brain Res 2016;1644:53-61. [Crossref] [PubMed]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192-205. [Crossref] [PubMed]
- Song JL, Priya N, Tektas SS, et al. microRNA regulation of Wnt signaling pathways in development and disease. Cell Signal 2015;27:1380-91. [Crossref] [PubMed]
- Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol 2016;99:141-9. [Crossref] [PubMed]
- Mariya T, Hirohashi Y, Torigoe T, et al. Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget 2016;7:26806-22. [Crossref] [PubMed]
- Justilien V, Regala RP, Tseng IC, et al. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One 2012;7:e35040 [Crossref] [PubMed]
- Liu H, Qin YR, Bi J, et al. Overexpression of matrix metalloproteinase 10 is associated with poor survival in patients with early stage of esophageal squamous cell carcinoma. Dis Esophagus 2012;25:656-63. [Crossref] [PubMed]
- Bikkavilli RK, Avasarala S, Van Scoyk M, et al. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene 2015;34:5317-28. [Crossref] [PubMed]
- Ramos-Solano M, Meza-Canales ID, Torres-Reyes LA, et al. Expression of WNT genes in cervical cancer-derived cells: Implication of WNT7A in cell proliferation and migration. Exp Cell Res 2015;335:39-50. [Crossref] [PubMed]
- Pierzynski JA, Hildebrandt MA, Kamat AM, et al. Genetic Variant Within the Wnt/B-Catenin Signaling Pathway as Indicators of Bladder Cancer Risk. J Urol 2015;194:1771-6. [Crossref] [PubMed]
- Urakami S, Shiina H, Enokida H, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/β-catenin signaling pathway. Clin Cancer Res 2006;12:383-91. [Crossref] [PubMed]
- Mao XW, Xiao JQ, Xu G, et al. CUL4B promotes bladder cancer metastasis and induces epithelial-to-mesenchymal transition by activating the Wnt/B-catenin signaling pathway. Oncotarget 2017;8:77241-53. [Crossref] [PubMed]
- di Martino E, Kelly G, Roulson JA, Knowles MA. Alteration of cell-cell and cell-matrix adhesion in urothelial cells: an oncogenic mechanism for mutant FGFR3. Mol Cancer Res 2015;13:138-48. [Crossref] [PubMed]
Cite this article as: Scheunemann D, Pradhan AK, Das SK, Sarkar D, Emdad L, Fisher PB. Wnt7a and miR-370-3p: new contributors to bladder cancer invasion. Biotarget 2018;2:14.


