
ALK-rearranged lung adenocarcinoma showing intra-bronchial protrusion: a case of actually peripheral origin with a rare spreading pattern
Introduction
Anaplastic lymphoma kinase (ALK)-rearranged lung adenocarcinoma is recognized to have a peripheral origin, the hallmarks of which are characterized by terminal bronchiolar and respiratory unit cells, including thyroid transcription factor-1 (TTF-1) positive cells; adenocarcinoma with ALK fusion is categorized into the so-called terminal respiratory unit (TRU)-type adenocarcinoma, which implies a peripheral origin of the tumor. However, several cases of allegedly central-type lung adenocarcinoma with ALK fusion diagnosed on computed tomography (CT) have been described in the published literature. Central-type tumors of the right lung are usually defined as those arising from the right main bronchus, lobar bronchi (B1+2+3), and segmental B1 bronchi. However, based on this criterion, we have encountered very few cases of central-type adenocarcinoma as well as central-type squamous cell carcinoma. Therefore, we have focused on the next generation of bronchial branching, i.e., subsegmental bronchi, in which tumors with similar characteristics as purely central-type arise. We encountered a case of lung adenocarcinoma with ALK fusion showing intra-bronchial protrusion and located at a subsegmental bronchus. To the best of our knowledge, such a case has never been previously reported. In this report, we present the first such case, along with molecular pathological analyses.
Case presentation
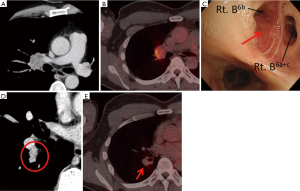
An asymptomatic 48-year-old Japanese man with elevated serum carcinoembryonic antigen (CEA) level (10.1 ng/mL) in medical examinations was referred to The Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan. He had no history of smoking or exposure to occupational or environmental carcinogens including asbestos. He had a family history of leukemia in his father but not of lung cancer. Chest CT scans showed a well-circumscribed 42-mm mass in the hilar region of the right upper lobe of the lung, which resembled a swollen lymph node with metastasis (Figure 1A). Positron emission tomography (PET)-CT showed increased fluorodeoxyglucose (FDG) uptake in the lesion (Figure 1B). Histopathological examination of the endobronchial ultrasound-guided transbronchial needle aspirate yielded a diagnosis of adenocarcinoma. During the bronchofiberscope procedure, we observed an elevated lesion that projected into the lumen of bronchus B6b~B6 entrance (Figure 1C), although we could not detect this lesion on CT or PET-CT. Transbronchial needle aspiration cytology and transbronchial biopsy of the lesion also showed adenocarcinoma. Repeat assessment of the CT radiograph showed a suspicious lesion in the S6 hilar region (Figure 1D) without FDG uptake on PET-CT (Figure 1E). This S6 hilar lesion was considered as the primary tumor, because no other candidate primary lesion was observed on thin-slice chest CT (1.25 mm thick). A diagnosis of primary lung adenocarcinoma in the S6 hilar region with lymph node metastases of the hilar region of the right upper lobe (#12u, #11s, and #10), cT1aN1M0 (cStage IIA), was established according to the 7th edition UICC TNM classification. Because of the metastatic swollen lymph node in the hilar region, right pneumonectomy with a curative intent was performed along with lymph node dissection.
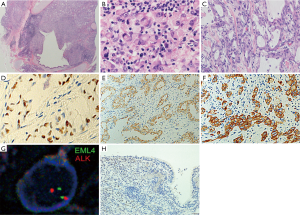
Macroscopically, a 13-mm primary lesion, which had displaced the bronchus B6b from outside, was found projecting into the bronchial lumen (Figure 2A). Histologically, the tumor was composed of a signet-ring cell component (Figure 2B) with a component of acinar structure with mucin (Figure 2C). Immunohistochemical staining was performed using the Leica Bond III automated system (Leica Biosystems Melbourne Pty Ltd., Australia). The tumor was positive for TTF-1 (clone: 8G7G3/1, DAKO, Carpinteria, USA, 1:1,000 dilution), Napsin A (clone: IP64, Leica Biosystems Newcastle Ltd., UK, 1:800 dilution), and ALK (clone: 5A4, Leica Biosystems Newcastle Ltd., 1:50 dilution) (Figure 2D,E,F). EML4-ALK rearrangement was detected by fluorescence in situ hybridization (FISH) fusion assay (Figure 2G). Epidermal growth factor receptor (EGFR) mutation was not detected. On the basis of histology, immunohistochemistry and FISH, the tumor was diagnosed as ALK rearrangement-positive acinar adenocarcinoma, moderately differentiated, with a signet-ring cell component, pT1aN2M0 (pStage IIIA) according to the 7th edition Union for International Cancer Control (UICC) TNM classification.
The patient was discharged from hospital without any complications. Adjuvant chemotherapy with cisplatin (CDDP) + vinorelbine (VNR) was administered. The patient is alive and has not shown any signs of recurrence 16 months after the surgery.
Discussion
Main clinicopathological characteristics of lung adenocarcinoma with ALK fusion include young age at onset, lack of a strong association with smoking, obvious acinar histology with mucin or signet-ring cells, and a TTF-1 cell lineage (1,2). However, our present case with ALK fusion is macroscopically centrally located and immunohistochemically TTF-1 positive, which suggests a peripheral origin. There have been several papers published for cellular origin and etiology of lung adenocarcinoma (3-7).
Almost all lung adenocarcinoma with ALK rearrangement is positive for TTF-1, and classified as a TRU type. We have never encountered a case of central-type lung adenocarcinoma with ALK fusion in our clinical experience. We examined the tumor location on CT and TTF-1 expression in 42 patients with ALK-rearranged lung adenocarcinomas resected at our hospital between 2005 and 2013. In this study, all tumors were of peripheral-type on CT (100%, 42/42) and all but one tumors were TTF-1-positive (97.6%, 41/42).
Several reports have described macroscopically central-type lung adenocarcinoma with ALK fusion diagnosed on CT; for example, central-type tumors comprised 57.4% (8) or 48.9% (9). However, these studies only used macroscopic classification based on CT and did not consider TTF-1 expression. Furthermore, the diagnostic criterion for central type has not been described in detail in these studies. Although a recent report showed the high-resolution CT features of ALK-rearranged lung adenocarcinoma (10), there are no reports that discuss the location of this tumor. To the best of our knowledge, ALK-rearranged lung cancer projecting into the bronchial lumen on bronchofiberscopy has not yet been described.
TTF-1 regulates normal peripheral lung functions, and is necessary for lung morphogenesis (3). TTF-1 not only activates surfactant protein genes in type-II pneumocytes and Clara cells (11), but also plays a key role in the differentiation and function of type-II pneumocytes in the fetus (12). After birth, TTF-1 was found to be selectively expressed in type-II pneumocytes and non-ciliated respiratory bronchial cells in the conducting regions of the lung (13).
These findings are consistent with the hypothesis that tumor TTF-1 expression points toward the peripheral origin of the tumor. In our case, nuclear TTF-1 immunostaining was detected only in the tumor cells, but not in the adjacent normal bronchial epithelial cells, which were compressed by the tumor (Figure 2H). This suggests that the tumor cells did not originate from the bronchial epithelial cells, constituting convincing evidence that the tumor did not originate from the central respiratory epithelium.
Our case promotes awareness about patients with ALK-rearranged lung adenocarcinoma. The tumor can be classified into TRU-type on the basis of positive immunostaining of TTF-1, even if it appears to be of central type based on macroscopic characteristics. Cases of macroscopically central-type ALK-rearranged lung adenocarcinoma (8,9) may be of TRU-type, based on cellular origin. Detailed investigations are required to elucidate the location of previously reported lung adenocarcinoma with ALK fusion by careful confirmation of bronchial branching and TTF-1 expression.
Acknowledgments
We thank Dr. Manabu Takamatsu for technical assistance in the FISH procedure.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/biotarget.2017.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol 2002;26:767-73. [Crossref] [PubMed]
- Hashimoto T, Tokuchi Y, Hayashi M, et al. Different subtypes of human lung adenocarcinoma caused by different etiological factors. Evidence from p53 mutational spectra. Am J Pathol 2000;157:2133-41. [Crossref] [PubMed]
- Yatabe Y, Kosaka T, Takahashi T, et al. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol 2005;29:633-9. [Crossref] [PubMed]
- Takeuchi T, Tomida S, Yatabe Y, et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol 2006;24:1679-88. [Crossref] [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Non-terminal respiratory unit type lung adenocarcinoma has three distinct subtypes and is associated with poor prognosis. Lung Cancer 2014;84:281-8. [Crossref] [PubMed]
- Yoon HJ, Sohn I, Cho JH, et al. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine (Baltimore) 2015;94:e1753 [Crossref] [PubMed]
- Yamamoto S, Korn RL, Oklu R, et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 2014;272:568-76. [Crossref] [PubMed]
- Nakada T, Okumura S, Kuroda H, et al. Imaging characteristics in ALK fusion-positive lung adenocarcinomas by using HRCT. Ann Thorac Cardiovasc Surg 2015;21:102-8. [Crossref] [PubMed]
- Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol 1994;14:5671-81. [Crossref] [PubMed]
- Benlhabib H, Guo W, Pierce BM, et al. The miR-200 family and its targets regulate type II cell differentiation in human fetal lung. J Biol Chem 2015;290:22409-22. [Crossref] [PubMed]
- Ikeda K, Clark JC, Shaw-White JR, et al. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 1995;270:8108-14. [Crossref] [PubMed]
Cite this article as: Noma D, Inamura K, Matsuura Y, Ninomiya H, Ichinose J, Nakao M, Mun M, Ishikawa Y, Okumura S. ALK-rearranged lung adenocarcinoma showing intra-bronchial protrusion: a case of actually peripheral origin with a rare spreading pattern. Ann Clin Oncol 2017;1:15.


