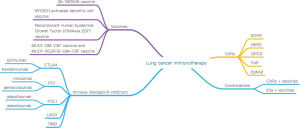
The revolution of lung cancer treatment: from vaccines, to immune checkpoint inhibitors, to chimeric antigen receptor T therapy
Background
Lung cancer is the leading cause of cancer death among men and the second leading cause of cancer death among women worldwide (1,2). For several years, surgery, chemotherapy (including neoadjuvant chemotherapy), radiotherapy and targeted therapy have been widely used clinically (3). Nevertheless, the overall survival (OS) rate of lung cancer still remains unacceptable (4). Whereas, with the leap and bounce of lung cancer immunotherapy, we predict that the scientific turning point is coming. So it is fair to conclude that treatment with immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T (CAR-T) cell immunotherapy will become the backbone of lung cancer therapy in the near future (5,6).
In the review, we categorized the progress in pulmonary carcinoma therapy into three parts, including the closely completion of vaccines, the galloping of ICIs and the initial exploration of CAR-T therapies. (I) Clinical results of vaccines are far from impressive, in that most of the related works have been done about one decade ago. In most cases, even no significant difference of the efficacy between vaccines and chemotherapy was observed, however, the toxicities and side effects of vaccines can be neglected. In addition, in order to treat precancerosis or prevent relapsed tumor, taking vaccines is a perfect choice. (II) With respect to ICIs, their efficacy has convinced numerous scientists to conduct more profound researches. Ipilimumab [anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)], a generation 1 inhibitor, was early approved by the US Food and Drug Administration (FDA) in 2011. Currently, the generation 1 inhibitors are under study in combination with generation 2 inhibitors or CAR-T therapies. Generation 2 inhibitors, the anti-programmed death 1 (PD1) agents, proved to have approximately a 20% object response rate (ORR). All anti-PD1 inhibitors, including nivolumab and pembrolizumab have all been approved by FDA in 2015 and 2014 respectively. Furthermore, scientists are attempting to find out whether generation 2 inhibitors can be used as first-line therapy (7). Trials on generation 3 inhibitors, the anti-programmed cell death-1 ligand-1 (PDL1) drugs, are still ongoing. Besides, the selection of biomarker and immune related adverse events are now the advanced research hotspots. (III) Breakthroughs in CAR-T therapy on hematologic malignancies have been achieved about half a decade ago, albeit, studies on solid tumors have just been initiated. To date, all published trials on lung cancer only include four preclinical studies and one phase I study. Based on data obtained from www.clinicaltrials.gov (up to January 2017), nine phase I or phase I/II studies are currently on the way. The initial clinical outcomes manifest that CAR-T therapy has a more robust antitumor effect than ICIs and vaccines, and the perfect example is that epidermal growth factor receptor (EGFR)-CAR T therapy benefit patients who could not respond to EGFR tyrosine kinase inhibitors. So what is the future of lung cancer therapy? Vaccines, ICIs and CAR-T therapies provide an instructive answer.
Thus far, multiple reviews have summarized the efficacy of vaccines in treatment of lung cancer (8,9). Likewise, outcomes and toxicities of lung cancer related ICIs have also been reviewed (10-12). However, there are no reviews with regard to CAR-T therapy on lung cancer.
In this review, we attempt to have a retrospective assessment of the freshly minted therapies, where the lung cancer therapy has been evolving from antitumor vaccines and ICIs to CAR-T therapies.
Vaccines
Compared with CAR-T therapy and ICIs based therapy, vaccines seem to develop less rapidly. In recent years, there is no remarkable breakthrough. Antitumor vaccines are designed to trigger a robust T cell response, rather than affecting the mechanism of immunosuppression. Different with CAR-T or ICIs therapy, the mechanism of vaccine is far from complex. These vaccines elicit antitumor responses against tumor related antigens. It directly stimulates the patient’s own immunological surveillance. Thus, antitumor vaccines have been applied more successfully in the case of less immunosuppression. As is illustrated in Table 1, vaccines alone usually have a mild antitumor activity. Compared with chemotherapy, even, statistically significant differences of OS or progression-free survival (PFS) cannot be seen (13), while, dual-target or multi-target vaccines can have a comparatively robust antitumor effect. Moreover, when treating precancerosis, or preventing relapsed tumor, injecting vaccines is a perfect selection. Another advantage of vaccines over other therapies is the slight toxic. More related discussion and data can be seen in the detailed meta-analysis (8).
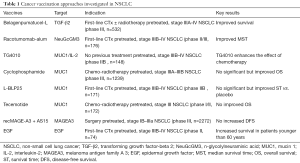
Full table
In the future, two main trends will lead the development of antitumor vaccines. The first one is more researches on dual-target or multi-target vaccines. A typical example is BI-1361849 vaccine, which include six targets in all. Latest vaccines, such as MUC1-granulocyte-macrophage colony stimulating factor (GM-CSF) vaccine and MUC1-VEGFR2-GM-CSF vaccine, are also targeted multiple antigens. The second trend is that more studies will be focused on the combination between vaccines and other immunotherapies, because features of vaccine, ICIs and CAR-T are different. At present, preclinical studies on vaccines combining with anti-PD1 agents are emerging, while only a small amount of clinical trials were published. With respect to combinations between vaccines and CAR-T therapy, a few scientists are making efforts preliminarily. Below, we discussed different kinds of vaccines and combination of vaccines.
EGFR belongs to the receptor tyrosine kinases (RTKs) family. It is expressed in 40% to 80% of non-small-cell lung carcinoma (NSCLC) cases. Epidermal growth factor (EGF), EGFR’s major ligand, can activate EGFR. Recombinant human EGF conjugated to a carrier protein (CIMAvax EGF) vaccines, developed by Cuban scientists, can raise antibodies targeting EGF and hence reduce the concentrations of EGF in the blood (14). Recently, a randomized phase III study was conducted in treatment with advanced NSCLC. Among 405 patients with stage IIIB/IV NSCLC, long-term vaccination turned out to be safe, in that most of adverse events were grade 1 or grade 2. A longer median survival time (MST) of 10.83 months was observed compared with the control arm (10.8 vs. 8.9 months). A higher MST of 14.66 months was reported when treated patients with high EGF concentration (15). Another research assessed the efficacy of the CIMAvax EGF vaccines in treatment of NSCLC patients. The 2-year OS rate and PFS rate was 20.7% and 30.5% respectively, and a median OS of 13 months was noted (16). A phase I/II trial (NCT02955290) is currently recruiting patients, studying the best dose and side effects of CIMAvax EGF vaccine combined with nivolumab in treating patients with stage IIIB–IV NSCLC.
MUC1-GM-CSF vaccine is a newly developed dual-target vaccine for lung cancer. MUC1, overexpressed aberrantly in lung tumors, can be a potential target in the use of lung cancer immunotherapy (17). GM-CSF, which worked as an adjuvant, is able to stimulate hematopoietic progenitors’ proliferating, differentiating and maturing (18,19). Therefore, its existence often marks the infiltration and metastasis of tumor cells. In a preclinical study, researchers tested the DNA vaccine based on MUC1-GM-CSF fusion gene in a mouse model, and compared with MUC-1 vaccine, GM-CSF vaccine and empty vector. As a result, significant decline of tumor weight and tumor growth rates in the group treated with MUC1-GM-CSF vaccine was observed (20). Vascular endothelial growth factor receptor 2 (VEGFR-2) belongs to the VEGF-family, expressed in newly born endotheliocytes of vessels. Another similar preclinical a study, was conducted in 2016, to evaluate the synergistic efficacy of MUC1-VEGFR2-GM-CSF DNA vaccines in comparison with MUC1 or VEGFR2 alone and MUC1-VEGFR2 vaccines in tumor-bearing mouse model. The enhanced inhibition of tumor growth and significant weight loss of tumor was showed in MUC1-VEGFR2-GM-CSF DNA vaccines treatment group (21). In conclusion, the synergistic antitumor efficacy of MUC1-VEGFR2-GM-CSF vaccines is robust.
BI-1361849, also named as CV9202, is a therapeutic self-adjuvanting mRNA vaccine. It targets six NSCLC-associated antigens (NY-ESO-1, MAGEC1, MAGEC2, 5 T4, survivin and MUC1). In a phase Ib trial, 26 patients with stage IV NSCLC were recruited to test RNActive cancer vaccine BI-1361849 combined with local radiotherapy. One confirmed partial remission was seen in a patient on maintenance pemetrexed. In conclusion, BI-1361849 can be safe in combination with local radiotherapy and maintenance pemetrexed treatment (22).
To date, quantities of studies concerning combinations of vaccines and other therapies are ongoing (23). Firstly, vaccines complement ICIs based therapies. Vaccines are able to induce the activation of T cells, while ICIs agents are likely to maintain the activity of T cells. A few preclinical studies have proved its efficacy in mice by now (24-28). And combinations of vaccines and ICIs based therapies had been proved to enhance OS and PFS in the treatment of lung cancer (8). Secondly, vaccine-chemotherapy combinations are also effective. Due to the robust killing efficacy of chemotherapeutics, it improved the microenvironment of tumor and spares much more time for vaccines to activate T cell responses. So many studies tended to examine vaccine-chemotherapy combinations (29-32). Thirdly, vaccines can enhance the antitumor effect of CAR-T cells (33). Relative studies on other cancers such as myeloma are currently on the way (34,35). Nevertheless, data related to lung cancer are now absent. Therefore, we hypothesize that large amount of studies will be started as the CAR therapy matures.
ICIs based therapy
ICIs based therapy, which is a vital part of immunotherapy, is revolutionizing the treatment of lung cancer. Astoundingly, Science magazine declared the immunotherapy as the breakthrough of 2013 owing to the prosperity of ICIs based therapy (36). So far, a few anti-PD drugs have been approved in many countries and regions, including US, Europe, Japan and so on (37).
In contrast with the other immunotherapies, the mechanism of immune checkpoint blockade is unique. Through blocking vital regulation of immune system, ICIs activate the T cells and augment their population, and T cells subsequently infiltrate and wipe out the tumor cells. As a result, the immune checkpoint blockade based agents have at least three features. Firstly, ICIs do not directly activate the immune system to attack tumor cells, but to eradicate the immune checkpoint blockade. So, ICIs are not specific for the type of cancer. And the immune response is universal, which independents of patients’ history of cancer or personal tumor-specific antigens. Secondly, unlike CAR-T therapy where the expression of target really occurs, the ongoing T cell response after injections of ICIs, rather than PD1 expressions, is the key factor. Thirdly, this treatment leads to durable responses, whose effect can remain even over a decade (38).
A consensus review published had a sound grip of the field of ICIs, and the writer Axel Hoos categorized the development of ICIs based therapies into three generations (39). Generation 1 consists of ipilimumab (anti-CTLA-4) and sipuleucel-T, which were approved by FDA on the basis of several randomized phase III clinical trials. Nevertheless, the failure of sipuleucel-T's mass production prevented it from becoming a commercial success. In recent three years, the generation 2, which includes PD1, has been progressing by leaps and bounds. Surprisingly, the PD1 based therapy, already approved by FDA, has been widely applied in routine clinical practice. It can be said without exaggeration that anti-PD1 therapy has been rapidly established as a widely used second-line treatment for lung cancer. Compared with chemotherapy, a stronger efficacy and less toxicity were noted based on the reported data. Combining anti-PD1 agents with chemotherapy also been proved to result in better ORR and OS rates. In 2016, multiple studies attempted to find out whether ICIs are more appropriate to be used in first-line therapy than platinum-doublet chemotherapy. Notably, the third generation, which contains atezolizumab (Tecentriq) and durvalumab (MEDI4736) that are both agents blocking PDL1, has aroused large quantities of scientists’ interest. The PDL1 molecule is essential for tumor-mediated immune evasion. Though anti-PDL1 therapy is still in the state of phase I/II trials, high chances are that it will be approved for the treatment of lung cancer. On account of data reported so far, we can safely arrive at the conclusion that the generation 3 can be the most competitive. Latest breakthroughs include relation between mutations and resistance to PD-1 blockade (40), a new way to handle the anti-PD drug-resistance (41), the reason why response rates vary in patients (42).
CTLA-4, also named CD152, is a protein receptor, which is expressed in activated T cells and regulatory T cells (Tregs). It plays an important role in regulating immune responses as an immune checkpoint. Ipilimumab is a human IgG1 anti-CTLA-4 monoclonal antibody. Likewise, tremelimumab is a fully human monoclonal antibody against CTLA-4. About one semi-decade ago, randomized phase III trials of ipilimumab and tremelimumab are successful (39). As a consequence, in 2011, ipilimumab was approved by FDA in treatment of metastatic melanoma (43). Now, researcher has focused on the synergistic effects of ipilimumab or tremelimumab in combination with other methods (such as PD1/PDL1 inhibitors). Moreover, the combination therapy proves to have better therapeutic effects than monotherapy (44,45).
PD1 inhibitors
PD1, termed as CD259, is another immune checkpoint receptor expressed in T cells, blocking T cell activity in parenchyma. It has two ligands, including PDL1 and PDL2 (B7-H1 or CD273), located on the surface of antigen presenting cells and tumors. PD1/PDL1 had been proved to inhibit activating of T cells. Though related tests and studies are currently absent, PD1/PDL2 seems to induce the similar effects (7). Compared with CTLA-4, an advantage of PD1 is its expression in other immune related cells, including B cells and NK cells, hence resulting in an enhanced stimulation of antibody production. Due to its features, quantities of studies endeavored to explore on the efficacy and safety of PD1 inhibitors such as nivolumab and pembrolizumab. Thus far, based on established data, an approximate ORR of 20% associated with PD1 monotherapy was reported. Higher ORR and lower toxicities of PD1 inhibitors have aroused the interest of numerous scientists.
Nivolumab (Opdivo) is a human IgG4 anti-PD1 monoclonal antibody, which can activate host immune system. FDA approved it in March 2015 for the treatment of metastatic squamous NSCLC based on the promising results of the CheckMate 017 (CM017) clinical trial (46). Excitingly, it was the first approved immunotherapy of squamous cell lung cancer (47). In 2015, multiple studies tended to evaluate the efficacy and safety of nivolumab, and find that nivolumab-based therapy produced durable responses and great survival rates in patients with NSCLC (48). In a phase I trial, median OS in patients with heavily pretreated NSCLC was 9.9 months at the 3 mg/kg dose (49). The result of other phase I study manifested that the ORR was 18% (95% CI, 11–29), under the condition that patients were injected at doses of 0.1–10 mg/kg once every 2 weeks (50). In 2016, a phase I/II trial (NCT01928394) showed that 98 patients with small-cell lung cancer (SCLC) was treated with nivolumab 3 mg/kg, and the median follow-up for patients was 198.5 days (51).
In comparison with other therapies, it seems fair to reach the conclusion that nivolumab is much more efficient based on a bunch of established researches. First of all, in contrast to chemotherapy, nivolumab was associated with significantly better OS, ORR and PFS in a randomized, open-label, international, phase III study (52). CheckMate 026 (NCT02041533) is examining nivolumab versus standard platinum-based doublet chemotherapy (PT-DC) in patients with stage IV or recurrent PD1-expression NSCLC who never received previous chemotherapy. Another trial, CheckMate 227 (NCT02477826) was initiated in order to investigate nivolumab and nivolumab combined with ipilimumab versus standard PT-DC with or without nivolumab. Secondly, the relationship between tumor PD1 expression and efficacy of agents are sufficient in a phase I, multi-cohort, Checkmate 012 trial (53). Thirdly, comparisons concerning the efficacy of nivolumab in treatment of SCLC versus NSCLC are now absent, and relevant studies are ongoing.
Nivolumab, coming of age, has been gradually applied. Thereby, the safety-related reports are numerous. A 2016 study reported two cases of NSCLC showing pseudoprogression during the nivolumab treatment (54). According to multiple reports, nivolumab can also potentially induce immune thrombocytopenia (55), psoriasis and psoriatic arthritis (56) or severe akathisia symptoms (57) in a patient with advanced lung cancer, acute demyelinating polyneuropathy in a patient with metastatic NSCLC (58), immune related pancreatitis in a patient with recurrent lung adenocarcinoma (LAD) (59), “disease flare” in a patient with stage IIB LAD (60), organizing pneumonitis in a patient with lung sarcomatoid carcinoma (61), relapse of morphea in a patient with LAD (62). More details of infusion-related adverse events (IRAEs) are discussed below.
Pembrolizumab (MK-3475 or Keytruda) is a humanized antibody against PD1 receptor. On the basis of the available data, some aspects of pembrolizumab are striking. First, with respect to the efficacy of pembrolizumab, a pivotal 2015 phase I study assigned 495 advanced NSCLC patients treated with pembrolizumab at a dose of either 2 or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks (63). Among all the patients, the ORR was 19.4%, while the median duration of response was 12.5 months. In 2016, multiple phase Ib or phase II studies manifested that pembrolizumab significantly prolongs OS for patients with PD1-positive advanced NSCLC (64-71). Second, with regard to the proper dose, a phase Ib trial characterized the relationship between different doses of pembrolizumab, indicating that no significant exposure dependency on efficacy or safety was identified across doses of 2 to 10 mg/kg (72). Third, a latest phase III study compared pembrolizumab-based therapy with standard PT-DC, with a result of better PFS and OS than chemotherapy (73). To put it in a nutshell, pembrolizumab is a mature agent. Impressively, on October 2, 2015, FDA granted accelerated approval for pembrolizumab (74).
PDL1 inhibitors
Atezolizumab (MPDL3280A) is a humanized monoclonal antibody of IgG1 isotype against PDL1. It is the first anti-PDL1 agent approved by the FDA for NSCLC, by virtue of the enhanced OS in a phase III trial (75). Another randomised, open-label, phase III trial (NCT02008227) tested the efficacy of atezolizumab in patients with squamous or NSCLC, suggesting that atezolizumab was associated with better OS in comparison with docetaxel (76). Analogously, a phase III trial comparing atezolizumab with chemotherapy proved that atezolizumab was applicable for the treatment of NSCLC (77), and atezolizumab plus chemotherapy in chemotherapy-naive patients with advanced NSCLC has robust efficacy (78). Clinical data obtained from three up-to-date trials concerning atezolizumab illustrate the broad potential of treatment on extensive stage small cell lung cancer (ES-SCLC) (79-81). With respect to the safety of atezolizumab, adverse events were less common in patients, compared with docetaxel (82). Interestingly, a phase II study pointed out the positive relation between the rate of PDL1 expression and OS (83).
Durvalumab (MEDI4736) is a fully human IgG1 antibody that is invented to inhibit PDL1, similar to atezolizumab. Compared with atezolizumab, nivolumab and pembrolizumab, the development and study of durvalumab is still being carrying on, in that almost all the clinical trials are in phase I. In other words, 2016 witnessed the preliminary advancement of durvalumab. In a recent phase I/II study, ORR was 25% when treating patients with advanced NSCLC (84). A phase I, open-label study aimed to examine the safety and antitumor activity of durvalumab in treatment of US patients with advanced NSCLC, and find that durvalumab was a safe and robust agent which was even effective in PDL1-negative patients (85). The safety of durvalumab also reported in the study (NCT01693562) showed that 50% of patients were confronted with durvalumab-related adverse events (86). Results of another phase II clinical study manifested a 5-year OS of 37% when treated in stage 3 NSCLC.
In the future, more efforts will be made from the following aspects. (I) Besides the known inhibitors, brand new immune checkpoint blockades will be explored, and the emerging inhibitors such as lymphocyte-activation gene 3 (LAG3) and T cell immunoglobulin- and mucin-domain-containing molecule 3 (TIM3) inhibitors will be thoroughly under study. (II) Optimum doses of anti-CDLA4 agents and anti-PD1 agents will be one of the key topics in the field of ICIs. (III) Investigations into ICIs-CAR therapy are now almost absent. Very few researches examine it in mouse model, and in the field of lung cancer, there is no related work regardless of its robust efficacy. Hence, more and more investigators will get involved in combining ICIs with CAR therapies.
LAG3, a new target, is a member of the immunoglobulin superfamily (IgSF), which has large quantities of impacts on T cell function (87). A preclinical trial managed to report that, in the experimental lung metastasis mouse models, MVA-BN-HER2 and/or anti-PD1 and anti-LAG3 dual checkpoint inhibition significantly reduced the size of tumor (88).
TIM3 is a transmembrane protein, which is T helper 1 (Th1) specific regulator of macrophage responses. Though researches about TIM3 are preliminary, the great potential in this field has generated a large amount of interest. A review titled “Immunotherapy: PD1 says goodbye, TIM3 says hello” published seems to predict the promising future of this therapy as its title manifests (89). Recently, preclinical trials revealed that TIM3 upregulation was observed in the tumor microenvironment in two fully immunocompetent mouse models of NSCLC (90). Currently, a phase I/II trial (NCT02608268) is on the way, testing anti-TIM3 monoclonal antibody along with PD1 inhibitor in patients with advanced solid malignancies, including NSCLC. Likewise, a phase I trial (NCT02817633) of TSR-022, an anti-TIM3 monoclonal antibody, is recruiting patients with NSCLC.
In recent 3 years, combination ICIs therapy, as a hot spot of this field, has been investigated by large quantities of studies. With the assistance of combination immunotherapies, durable responses have been obtained and OS and PFS of patients were significantly prolonged. In particular, success of nivolumab plus ipilimumab combination immunotherapy led to the approval by FDA owing to CheckMate 067 trial results (91). Whereas, some studies reported that combining anti-PD1 agents with anti-CTLA-4 agents or EGFR-tyrosine kinase inhibitors (TKIs) may not have significantly better results than anti-PD1 monotherapy. Recently, according to an important study published by Nature Medicine, Moynihan et al. combined four kinds of agents: anti-PD1 drugs, an antigen-targeting antibody, a recombinant IL-2 and a robust vaccine, and synergetic antitumor efficacy was observed (92). Additional data, another influential research published by Nature Medicine reported that focal adhesion kinase (FAK) inhibitors (VS-4718) plus checkpoint immunotherapy was much more efficient than monotherapy (93). In the near future, high chances are that combination immunotherapy will be used as the first-line therapy by virtue of its great clinical benefit.
Encouraging results of anti-PD1/PDL1 plus chemotherapy were reported, and toxicity was acceptable. (I) With respect to nivolumab (anti-PD1) plus chemotherapy, a phase I, multi-cohort study was aim to test nivolumab monotherapy versus combination therapy. A better ORR, PFS and OS were observed than PT-DC alone. And the grade 3/4 IRAE rate of 45% was seen (n=56) (94). Likewise, a phase Ib study reported a median PFS of 6.28, 9.63 months, not reached, and 3.15 months was observed among 4 arms (n=6 respectively). Higher chances of skin toxicities and hepatic toxicities were reported than chemotherapy or nivolumab alone, albeit, IRAEs were all mild (95). (II) Concerning atezolizumab (anti-PDL1) plus chemotherapy, multiple studies have been initiated, including IMpower130 (NCT02367781) (96), IMpower131 (NCT02367794) (97), IMpower132 (NCT02657434) (98) and IMpower133 (NCT02763579) (81).
Recently, an influential study reported that anti-PD1 drugs plus systemic chemotherapy (SC) can enhance antitumor efficacy in glioblastoma (GBM) (99). This trend may lead the future study in the field of lung cancer immunotherapy.
Data related to the efficacy of combination ICIs therapies are sufficient, especially nivolumab (anti-PD1) plus ipilimumab (anti-CTLA-4). The characteristics of nivolumab and ipilimumab account for the popularity of this combination immunotherapy. Based on established studies, anti-CTLA-4 agents will push T cells into tumors. As a result, the population of T cells will be enlarged, thereafter inducing PDL1-expression in the microenvironment. Hence, if anti-PD1 or anti-PDL1 agents are simultaneously used, more promising results will be possible (38). Also, on the basis of large quantities of data, CTLA-4 inhibitors, including ipilimumab, usually deliver relative poor response rates but low toxicities. On the other hand, nivolumab, a PD1 inhibitor, is able to have high response rates in most occasions. Thereby, combination immunotherapy works fairly well. Researches indicated nivolumab plus ipilimumab therapy in the first-line setting generated antitumor activities with higher response rate compared with nivolumab monotherapy (51,100). It made patients have better ORR in treatment of lung cancer, and the rate of IRAE is normal (101). Another example is durvalumab (anti-PDL1) plus tremelimumab (anti-CTLA-4), but related studies are comparatively fewer (102).
Besides anti-PD1/PDL1 plus chemotherapy and anti-PD1/PDL1 plus anti-CTLA-4, durvalumab (anti-PDL1) plus OX40 (NCT02221960), nivolumab (anti-PD1) plus lirilumab (NCT01714739), nivolumab (anti-PD1) and ipilimumab (anti-CTLA-4) plus radiotherapy (NCT02046733) cisplatin and etoposide plus thoracic radiotherapy followed by nivolumab or placebo (NCT02768558) are ongoing (103,104).
Notably, anti-PD agents plus CAR-T therapies has also aroused interest of investigators. In a preclinical study, investigators found that CAR-T cells secreted anti-PD-L1 antibodies more effectively, regressing renal cell carcinoma (105). It is fair to predict that more highly promising achievements will be made in the near future.
CAR-T cell immunotherapy therapy
With the ultimate goal of enhancing antitumor responses which is mediated by immune system, the adoptive cell therapy (ACT) was born, containing tumor-infiltrating lymphocyte (TIL) therapy (106), γδ T cell therapy (107), natural killer (NK) cell-based therapy (108), cytokine-induced killer (CIK) cell-based therapy (109) and CAR-T therapy. Originated from TIL immunotherapy, CAR is composed of three parts, including an extracellular antigen-target domain, a transmembrane domain and an intracellular signaling domain. The extracellular domain, in the simplest form, consists of a single-chain variable fragment (scFv), which is derived from the variable heavy and variable light chains of an antibody and used to target the tumor-associated antigen (TAA). The transmembrane domain anchors the CAR to the cell membrane. And the intracellular domain is composed of signaling domains that is essential for activation of T cells.
To date, CARs have developed to the third generation. The initial generation of CAR is composed of a single intracellular CD3ζ chain. The comparatively simple structure results in the anergy of T cells, because CD3ζ chain merely delivers activation signal 1 to T cells, thereby having a low overall expansion and antitumor ability (110-112). Further advancements of the CAR, known as the second generation, have included the addition of a co-stimulatory signaling chain on the basis of a CD3ζ domain. Thus, the receptor provides both a signal 1 and signal 2 to induce the activation of T cells. The latest co-stimulatory chains that is being studied includes CD28 (111,113,114), 4-1BB (115,116), OX40 (117), CD27 (118), ICOS (119) and so on. The co-stimulatory domains vary in their attributes, such as the ability to confer cytokine secretion, cytotoxicity and proliferation. The third generation of CAR includes a CD3ζ domain and two co-stimulating domains, endowing CAR with enhanced activity, persistence and great antitumor efficacy. February 06, 2017 was a “big day”, witnessing the approval of the first off-the-shelf CAR product UCART123 by FDA. Recently, major breakthroughs are emerging. An important study had an in-depth study on exhausted T cells in cancer, making CAR-T therapy more promising (120). Scientists found that S-2-hydroxyglutarate regulated CD8+ T-lymphocyte fate, providing a new strategy to improve persistence of CAR-T cells (121). A study engineered T cells with dual-receptor and made CAR-T cells to recognize targets more precisely (122). Another study used double synNotch receptors in one T cell, allowing flexible user-customized extracellular cues (123).
There are several advantages of CAR. (I) In comparison with T cell receptor (TCR), CARs can avoid being constrained by major histocompatibility complex (MHC) specificity according to a fundamental 1990 research (124). As a result, CARs are likely to be applied to a wider range of patients than TCRs (125). (II) In CAR-T cells, a receptor was combined with T cell populations, allowing CAR-T cells to target at almost all the tumors. (III) Proteins, glycoproteins and glycolipids can be used as potential targets. (IV) CAR-T cells have a robust overall antitumor ability and greater persistence compared with normal T cells (126). However, there is no denying that the structure of CAR has a limitation that the intracellular antigens cannot be targeted, such as the MAGE family and NY-ESO1 (127). When it comes to the safety of this therapy, CAR T cells are likely to elicit potential toxicities, such as cytokine release syndrome (CRS), neurological toxicity, on-target/off-tumor recognition, anaphylaxis, insertional oncogenesis, graft versus host disease and off-target antigen recognition (128,129). And lots of studies have been initiated in order to manage the toxicity, including pharmacological immunosuppression, suicide genes, elimination genes and targeted activation (130-132).
Conspicuous clinical success observed in CAR-T based therapy has generated the growing interest (133) in the field of haematological malignancies, especially B-cell acute lymphoblastic leukemia (B-ALL) (134,135), chronic lymphocytic leukaemia (CLL) (136,137), multiple myeloma (MM) (138) and non-Hodgkin lymphoma (NHL) (139). Nevertheless, the majority of the studies concerning solid tumors are at the preclinical state or early phase clinical trials (140), indicating that CAR-T cell therapy for solid tumors will be progressing by leaps and bounds in the near future. In addition to lung tumor, multiple studies are concentrating on the treatment of prostate cancer (141-143), pancreatic cancer (144,145), mesothelioma (146), glioma (147), neuroblastoma (148), melanoma (149), sarcoma (150), GBM (151-155) and a growing list of other malignancies. Simultaneously, the researchers focused on solid tumors may be confronted with more challenges, and there are at least three main rationales for this puzzle. Firstly, the micro-environment of solid tumors may be significantly more immunosuppressive than B-ALL. Secondly, high chances are that the so-called ‘on-target, off-tumor’ toxicity may have negative effects on the antigen selection, because the immunotherapeutic target can also be expressed in normal tissues. Hence, it is more difficult to find an effective potential target, requiring the previous study of new antigens and the development of preclinical models (156). Thirdly, due to higher antigen heterogeneity in solid tumors, the selection of antigen becomes less possible (112).
At present, researches of lung cancer CAR-T therapy are still in the initial stage. Investigators focused on finding new potential targets and conducting clinical trials based on previous preclinical studies. Thus far, all published researches in the field of lung cancer only include four preclinical studies and one phase I study. Furthermore, there are nine ongoing trials, most of which are phase I trials. In Table 2, the ongoing phase I/II trials related to CAR-T therapy were summarized. The information in this table came from www.clinicaltrials.gov (up to January 2017). Simultaneously, new potential targets also exist. Targets including Tspan8, MUC1, CD151, CD146, LRP1 are promising (157), and encouraging results of these targets have been observed in other cancers, especially MUC1 (158-160). Scientists including Posey et al. recently provided insights and proved it (160). It is fair to predict that, in the near future, more investigators will get involved in testing the new targets.

Full table
EGFR is a transmembrane glycoprotein, which is also referred to as human epidermal receptor 1 (HER1). EGFR plays an important role in cell proliferation, survival, metastasis and tumor-induced vascularization (161). EGFR is expressed in normal epithelial cells and a lot of epithelial tissue deprived malignancies. Besides lung cancer, EGFR-CAR T therapy was applied to other kinds of cancers such as GBM (151). Compared with normal tissues, EGFR tends to be a promising therapeutic target because of the significant elevation of low-affinity and high-affinity of binding sites in lung tumors.
In 2013, a preclinical study firstly tested EGFR-CAR T cells in the A549 advanced lung cancer model. Consequently, EGFR-CAR T cells eliminate almost all tumor cells compared with other groups. Also, weights of lung significantly decreased in the group of EGFR-CAR T compared with all other groups (162). This study provided a basis for clinical trials of EGFR-CAR T. When it comes to the safety of EGFR-CAR T, no obvious fluctuating of cytokines and few adverse events were observed in mice (162). Thereafter, a 2016 phase I clinical trial (NCT01869166) proved the safety and feasibility of CAR-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed or refractory NSCLC. In their study, 6 women and 5 men with advanced relapsed or refractory NSCLC took part in, and the EGFR expression was over 50%. The EGFR-CAR T cells were produced from peripheral blood, with the result that a median of 29.28% of T cells from patients expressed the CAR. In other words, the specific toxicity of EGFR-CAR T cells from patients was able to act against EGFR-positive tumor cells. The certain toxicity of the EGFR-CAR T cells was observed after incubation with the EGFR-positive cells, HeLa and MCF7 Plus the infusions of EGFR-CAR T cells were well tolerated, indicating unserious cytotoxicity. Among 11 patients, a partial response of 2 patients was observed, while 5 stable diseases was reported, which range from two to eight months. Interestingly, the patients, where EGFR-TKIs were not effective, were able to benefit from the EGFR-CAR T therapy (163). This trial is the first clinical CAR study about lung cancer, and further studies on EGFR-CAR T cells are required.
Human EGF receptor 2 (HER2) is overexpressed in multiple malignancies, including breast cancer, lung cancer, ovary cancer, prostate cancer, brain cancer, colon cancer and ovarian cancer (156). In 2015, a phase I/II trial evaluated the efficacy of HER2-CAR T cells, where 19 patients with recurrent/refractory HER2-expressed sarcoma were recruited in. The median OS was 10.3 months, and the cells have great persistence without obvious toxicities (164). At present, an ongoing preclinical study is attempting to evaluate the efficacy of HER2-CAR T cells in vitro experiments in an established lung cancer model (165). Currently, a phase I/II study (NCT02713984) tested the efficacy of HER2-CAR T cells to confirm the ability of CAR T cells to eliminate HER2 positive cancer cells, including lung cancer cells. Another phase I/II research (NCT01935843) is currently recruiting participants with NSCLC in order to determine the safety and feasibility of HER2-CAR T cells.
Glypican-3 (GPC3) is affiliated to the family of heparin sulfate proteoglycans (HSPG), which are dependent on the cell surface by a glycosylphosphatidylinositol anchor. It has important effects on cellular growth, differentiation, and migration (166,167). GPC3 is over-expressed in many kinds of tumor cells, including lung tumor, and it is deficient in normal tissues. In addition to lung cancer, researches on CAR-T-GPC3 therapy for hepatocellular carcinoma are also under way (168-170).
In 2016, a preclinical study found that the antitumor affected generation 3 GPC3-CAR T cells in an established lung squamous cell carcinoma (LSCC) model. They firstly explored by immunohistochemistry (IHC) that GPC3 was expressed in LAD (3.33% positive) cases and LSCC cases (63.33% positive). However, GPC3 was not expressed in normal lung tissues. In the assay of a cytokine release, GPC3-CAR T cells released a strikingly increased amount of IFN-γ, IL-2, TNF-α, IL-4 and IL-10, indicating a strong activation of T cells. The results of cytotoxicity assay proved that GPC3-CAR T cells were capable of eradicate GPC3-positive cells. In LSCC models, GPC3-CAR T cells could eradicate almost all the growth of GPC3-positive cells. To put it in a nutshell, high chances are that GPC3-CAR T cells based therapy can be a promising therapeutic agent for the treatment of patients with LSCC (171). A phase I clinical trial (NCT02876978) is currently recruiting LSCC participants in order to examine the tolerance of GPC3-CAR T cells and the survival of the GPC3-CAR T cells in vivo.
Fibroblast activation protein-α (FAP) is a type 2 dipeptidyl peptidase, expressed in cancer-associated fibroblasts (CAFs) in most of the solid tumors, including lung tumors (172,173). In a preclinical study, researchers genetically modified a generation 2 CAR, which is specific for murine-FAP (mFAP) and human-FAP (hFAP). In lung cancer models, the cytotoxicity of mhFAP-CAR T cells against mFAP and hFAP was significant, compared with the non-transduced T cells. The trial manifested that mhFAP-CAR T cells could perfectly recognize and eradicate both hFAP-expressing and mFAP-expressing cells. In addition, mhFAP-CAR T cells had antitumor activity in both a loco-regional tumor model and a systemic tumor model. An application of FAP-CAR T cells tends to be a promising and feasible therapy (174).
Ephrin type-A receptor 2 (EphA2), composed of a mono-kinase domain and an ectocytic domain, is overexpressed in primary lung tumor cells. It belongs to the largest family of RTKs (175,176). In a preclinical trial, using A549 lung cancer models, EphA2-CAR T cells recognized and eradicated EphA2-expressed targets, and EphA2-CAR T cells enable bystander T cells to eradicate EphA2-expressed tumor cells. The potent antitumor efficacy of EphA2-CAR T cells was observed in mice, inducing a significant survival advantage (177). A combination of EphA2-CAR T cells plus FAP-CAR T cells was tested in another preclinical study of lung tumor. A median expression rate of 36.6% was reported in EphA2-CAR T cells. As a result, EphA2-expressed targets were recognized and eradicated by EphA2-CAR T cells, whereas the EphA2-negative cells stay uninjured. The combination of EphA2-CAR T cells plus FAP-CAR T cells had a better antitumor activity, and the difference between it and other therapies was significant. Thus, EphA2-CAR T combining with FAP-CAR T cells significantly enhanced overall antitumor activity (174).
With respect to CAR-T therapy, promising therapeutic targets are available, such as Tspan8, MUC1, CD151, CD146, LRP1, which are overexpressed in lung tumor cells. More preclinical studies will be initiated in mouse models in order to examine their efficacy. In particular, MUC-1 will be a promising target based on an influential research (160). In addition, results of nine ongoing phase I/II trials may be encouraging. Combining CAR-T therapy with vaccines, ICIs will be promising, and scientists have proved it in a similar way (92). This field is still young, but a lot of potential for progress and clinical improvements exists.
Summary
The immunotherapy for lung cancer in the review is summarized in Figure 1. The field of lung cancer therapy is undergoing revolutionary changes. New antitumor vaccines can prolong OS and PFS, and it is useful when treating precancerous lesion or preventing relapse tumor. Anti-PD1 agents may have roughly a 20% ORR, associated with significantly better OS and PFS than chemotherapy. Emerging ICIs, including PDL1, LAG3 and TIM3, will generate prolonged OS and PFS, though, more in-depth studies are required. Combinations between PD1/PDL1 inhibitors, CTLA-4 inhibitors and chemotherapies are under study, and the optimum dose remains unknown. Patients who haven't been pretreated with chemotherapy are likely to benefit more from ICIs. Preclinical studies on CAR-T in treatment lung cancer have produced preliminary achievements, including HER2-CAR T cells, GPC3-CAR T cells, FAP-CAR T cells and EphA2-CAR T cells therapies. Multiple phase I/II studies are continuing. In the future, scientist in the world will move ahead on the road of immunotherapy for lung cancer to bring more hope.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Duan J, Hao Y, Wan R, et al. Efficacy and safety of weekly intravenous nanoparticle albumin-bound paclitaxel for non-small cell lung cancer patients who have failed at least two prior systemic treatments. Thorac Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Watkins C, Huang X, Latimer N, et al. Adjusting overall survival for treatment switches: commonly used methods and practical application. Pharm Stat 2013;12:348-57. [Crossref] [PubMed]
- Davarpanah NN, Yuno A, Trepel JB, et al. Immunotherapy: a new treatment paradigm in bladder cancer. Curr Opin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Huang Y, Liang D, Liu J, et al. The Breakthroughs in Cancer Immune Checkpoint Based Therapy: a review of development in immune checkpoint study and its application. Comb Chem High Throughput Screen 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Decatris MP, O'Byrne KJ. Immune checkpoint inhibitors as first-line and salvage therapy for advanced non-small-cell lung cancer. Future Oncol 2016;12:1805-22. [Crossref] [PubMed]
- Dammeijer F, Lievense LA, Veerman GD, et al. Efficacy of Tumor Vaccines and Cellular Immunotherapies in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol 2016;34:3204-12. [Crossref] [PubMed]
- Mountzios G, Linardou H, Kosmidis P. Immunotherapy in non-small cell lung cancer: the clinical impact of immune response and targeting. Ann Transl Med 2016;4:268. [Crossref] [PubMed]
- Du L, Herbst RS, Morgensztern D. Immunotherapy in Lung Cancer. Hematol Oncol Clin North Am 2017;31:131-41. [Crossref] [PubMed]
- Cortinovis DL, Canova S, Abbate M, et al. Focus on Nivolumab in NSCLC. Front Med (Lausanne) 2016;3:67. [Crossref] [PubMed]
- Remon J, Chaput N, Planchard D. Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer. Curr Opin Oncol 2016;28:122-9. [Crossref] [PubMed]
- Yang L, Wang L, Zhang L. Immunotherapy for lung cancer: advances and prospects. Am J Clin Exp Immunol 2016;5:1-20. [PubMed]
- Khanna P, Blais N, Gaudreau PO, et al. Immunotherapy Comes of Age in Lung Cancer. Clin Lung Cancer 2017;18:13-22. [Crossref] [PubMed]
- Rodriguez PC, Popa X, Martinez O, et al. A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2016;22:3782-90. [Crossref] [PubMed]
- Cobian CO, Acosta Brooks SC, Feria FM, et al. P2.32: Survival Assessment of Non-Small Cell Lung Neoplasia Patients in Advanced Stage Treated With the CIMAvax-EGF Vaccine: Track: Immunotherapy. J Thorac Oncol 2016;11:S235-S236. [Crossref] [PubMed]
- Hossain MK, Wall KA. Immunological Evaluation of Recent MUC1 Glycopeptide Cancer Vaccines. Vaccines (Basel) 2016;4:E25. [Crossref] [PubMed]
- Hoeller C, Michielin O, Ascierto PA, et al. Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother 2016;65:1015-34. [Crossref] [PubMed]
- Becher B, Tugues S, Greter M. GM-CSF. From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016;45:963-73. [Crossref] [PubMed]
- Yang Z, Jiang D, Zhu Q, et al. The immunogenicity and anti-tumor effects of a lung cancer DNA vaccine harboring a MUC-1 and GM-CSF fusion gene. Brazilian Archives of Biology and Technology 2016.59.
- Ruan J, Duan Y, Li F, et al. Enhanced synergistic anti-Lewis lung carcinoma effect of a DNA vaccine harboring a MUC1-VEGFR2 fusion gene used with GM-CSF as an adjuvant. Clin Exp Pharmacol Physiol 2017;44:71-8. [Crossref] [PubMed]
- Hipp M, Sebastian M, Weiss C, et al. Abstract B072: Phase Ib trial of the RNActive cancer vaccine BI 1361849 (CV9202) and local radiotherapy in patients with stage IV non-small cell lung cancer (NSCLC) with disease control after first-line chemotherapy or during therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: Updated clinical results and immune responses. Cancer Immunol Res 2016;4:B072. [Crossref]
- van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016;16:219-33. [Crossref] [PubMed]
- Kim V, Foley K, Soares K, et al. Sequential treatment with a listeria-based vaccine and PD-1 blockade antibody improves survival in a murine model of pancreatic ductal adenocarcinoma. Hpb 2016;18:e45-6. [Crossref]
- Weir G, Hrytsenko O, Quinton T, et al. Multimodal therapy with a potent vaccine, metronomic cyclophosphamide and anti-PD-1 enhances immunotherapy of advanced tumors by increasing activation and clonal expansion of tumor infiltrating T cells. Cancer Res 2016;76:abstr 4903.
- Cappuccini F, Stribbling S, Pollock E, et al. Immunogenicity and efficacy of the novel cancer vaccine based on simian adenovirus and MVA vectors alone and in combination with PD-1 mAb in a mouse model of prostate cancer. Cancer Immunol Immunother 2016;65:701-13. [Crossref] [PubMed]
- Hu Y, Xia Y, Chen X, et al. Increasing the efficacy of cancer stem cell-based vaccine in the PD-L1/PD-1 blockade. Cancer Res 2016;76:abstr 3329.
- Hailemichael Y, Fu T, Woods A, et al. Abstract A031: Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. Cancer Immunol Res 2016;4:A031. [Crossref]
- Toleman MS, Herbert K, McCarthy N, et al. Vaccination of chemotherapy patients--effect of guideline implementation. Support Care Cancer 2016;24:2317-21. [Crossref] [PubMed]
- Welters MJ, van der Sluis TC, van Meir H, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016;8:334ra52. [Crossref] [PubMed]
- Tagliamonte M, Petrizzo A, Mauriello A, et al. Abstract A045: Inhibition of tumor growth by combination of metronomic chemotherapy and checkpoint inhibitor with a cancer vaccine. Cancer Immunol Res 2016;4:A045. [Crossref]
- Tagliamonte M, Petrizzo A, Tornesello ML, et al. Evaluation of novel metronomic chemotherapy and cancer vaccine combinatorial strategy. Cancer Immunol Res 2016;4:abstr B130.
- Slaney CY, Westwood JA, Beavis PA, et al. Eradication of large solid tumors in immunocompetent mice using dual specific CAR T cells and vaccination. Cancer Immunol Res 2016;4:abstr A104.
- Patel KK. Employing t cells for antigen presentation: role of NY-ESO-1+ T-APC vaccine in multiple myeloma. 2016. [Epub ahead of print].
- Patel K, Olivares S, Singh H, et al. Combination Immunotherapy with NY-ESO-1-Specific CAR+ T Cells with T cell Vaccine Improves Anti-Myeloma Effect. Am Soc Hematology 2016;128:3366.
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]
- Wang J, Yuan R, Song W, et al. PD-1, PD-L1 (B7-H1) and Tumor-Site Immune Modulation Therapy: The Historical Perspective. J Hematol Oncol 2017;10:34. [Crossref] [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Hoos A. Development of immuno-oncology drugs - from CTLA-4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235-47. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Benci JL, Xu B, Qiu Y, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016;167:1540-54 e12.
- Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35-44. [Crossref] [PubMed]
- Hazarika M, Chuk MK, Theoret MR, et al. U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab. Clin Cancer Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Zhan H, Yang Y, Zhao B, et al. Therapeutic effects and associated adverse events of PD-1 and CTLA-4 pathway inhibitors in the treatment of non-small-cell lung cancer: meta-analyses of clinical phase II/III randomized controlled trials. Int J Clin Exp Med 2017;10:1529-38.
- Aldarouish M, Wang C. Trends and advances in tumor immunology and lung cancer immunotherapy. J Exp Clin Cancer Res 2016;35:157. [Crossref] [PubMed]
- Kazandjian D, Suzman DL, Blumenthal G, et al. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist 2016;21:634-42. [Crossref] [PubMed]
- Aguiar PN Jr, Santoro IL, Tadokoro H, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy 2016;8:1011-9. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- de Mello RA, Veloso AF, Esrom Catarina P, et al. Potential role of immunotherapy in advanced non-small-cell lung cancer. Onco Targets Ther 2016;10:21-30. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer 2016;102:44-8. [Crossref] [PubMed]
- Bagley SJ, Kosteva JA, Evans TL, et al. Immune thrombocytopenia exacerbated by nivolumab in a patient with non-small-cell lung cancer. Cancer Treatment Communications 2016;6:20-3. [Crossref]
- Law-Ping-Man S, Martin A, Briens E, et al. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology (Oxford) 2016;55:2087-9. [Crossref] [PubMed]
- Abe J, Sato T, Tanaka R, et al. Nivolumab-Induced Severe Akathisia in an Advanced Lung Cancer Patient. Am J Case Rep 2016;17:880-2. [Crossref] [PubMed]
- Ur Rehman BS, Bhaumik S. Acute Demyelinating Polyneuropathy Caused by Nivolumab in a Man with Metastatic Non-Small Cell Lung Cancer. Journal of Gerontology & Geriatric Research 2016;5:302. [Crossref]
- Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer 2016;99:148-50. [Crossref] [PubMed]
- Chubachi S, Yasuda H, Irie H, et al. A Case of Non-Small Cell Lung Cancer with Possible “Disease Flare” on Nivolumab Treatment. Case Rep Oncol Med 2016;2016:1-3.
- Gounant V, Brosseau S, Naltet C, et al. Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer 2016;99:162-5. [Crossref] [PubMed]
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during Nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr 2017;108:69-70. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Ramalingam S, Hui R, Gandhi L, et al. P2.39: Long-Term OS for Patients With Advanced NSCLC Enrolled in the KEYNOTE-001 Study of Pembrolizumab: Track: Immunotherapy. J Thorac Oncol 2016;11:S241-2. [Crossref] [PubMed]
- Vizcarrondo F, Patel S, Pennell N, et al. Phase 1b study of crizotinib in combination with pembrolizumab in patients (pts) with untreated ALK-positive (+) advanced non-small cell lung cancer (NSCLC). Ann Oncol 2016;27:1291TiP.
- Chatterjee MS, Turner DC, Ahamadi M, et al. Exposure-response analysis of pembrolizumab in patients with advanced melanoma and non-small cell lung cancer enrolled in KEYNOTE-001, -002, and -006. Cancer Res 2016;76:abstr CT112.
- Herbst RS, Baas P, Perez-Gracia JL, et al. PD1. 06 (also presented as P2. 41): Pembrolizumab vs Docetaxel for Previously Treated NSCLC (KEYNOTE-010): Archival vs New Tumor Samples for PD-L1 Assessment. J Thorac Oncol 2016;11:S174-5. [Crossref] [PubMed]
- Kato T, Takahashi T, Yoshioka H, et al. KEYNOTE-025: Phase 1b study of pembrolizumab (pembro) in Japanese patients (pts) with previously treated PD-L1+ non-small cell lung cancer (NSCLC). Ann Oncol 2016;27:1221P. [Crossref]
- Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27:1291-8. [Crossref] [PubMed]
- Goodwin PM. First-Line Pembrolizumab Boosts Survival in Advanced Lung Cancer. Oncology Times 2017;39:39. [Crossref]
- Sul J, Blumenthal GM, Jiang X, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016;21:643-50. [Crossref] [PubMed]
- Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol 2016;27:LBA44. _PR. [Crossref]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Herbst RS, De Marinis F, Jassem J, et al. PS01. 56: IMpower110: Phase III Trial Comparing 1L Atezolizumab with Chemotherapy in PD-L1–Selected Chemotherapy-Naive NSCLC Patients. J Thorac Oncol 2016;11:S304-S305. [Crossref] [PubMed]
- Jotte RM, Socinski MA, Reck M, et al. PS01. 53: First-Line Atezolizumab Plus Chemotherapy in Chemotherapy-Naive Patients with Advanced NSCLC: A Phase III Clinical Program. J Thorac Oncol 2016;11:S302-3. [Crossref] [PubMed]
- Horn L, Reck M, Mok T, et al. PS01.57: IMpower133: a Phase I/III Study of 1L Atezolizumab with Carboplatin and Etoposide in Patients with Extensive-Stage SCLC: Topic: Medical Oncology. J Thorac Oncol 2016;11:S305-S306. [Crossref] [PubMed]
- Sequist L, Chiang A, Gilbert J, et al. Clinical activity, safety and predictive biomarkers results from a phase Ia atezolizumab (atezo) trial in extensive-stage small cell lung cancer (ES-SCLC). Ann Oncol 2016;27:1425PD.
- Horn L, Reck M, Mok TSK, et al. A Phase III study of atezolizumab with carboplatin plus etoposide in patients with extensive-stage small cell lung cancer (IMpower133). Ann Oncol 2016;27:1431TiP.
- Leventakos K, Mansfield AS. Advances in the Treatment of Non-small Cell Lung Cancer: Focus on Nivolumab, Pembrolizumab, and Atezolizumab. BioDrugs 2016;30:397-405. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Antonia S, Kim S, Spira A, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non-small-cell lung cancer. J Clin Oncol 2016;34:9029.
- Karakunnel JJ. O2-8-1Safety and efficacy of durvalumab (MEDI4736) plus tremelimumab in advanced non-small-cell lung cancer (NSCLC). Ann Oncol 2016;27:mdw521. 045.
- Antonia S, Rizvi N, Brahmer J, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Res 2016;4:abstr A047.
- He Y, Rivard CJ, Rozeboom L, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci 2016;107:1193-7. [Crossref] [PubMed]
- Foy SP, Sennino B, dela Cruz T, et al. Poxvirus-Based Active Immunotherapy with PD-1 and LAG-3 Dual Immune Checkpoint Inhibition Overcomes Compensatory Immune Regulation, Yielding Complete Tumor Regression in Mice. PLoS One 2016;11:e0150084. [Crossref] [PubMed]
- Romero D. Immunotherapy: PD-1 says goodbye, TIM-3 says hello. Nat Rev Clin Oncol 2016;13:202-3. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Bansal P, Osman D, Gan GN, et al. Recent Advances in Immunotherapy in Metastatic NSCLC. Front Oncol 2016;6:239. [PubMed]
- Moynihan KD, Opel CF, Szeto GL, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 2016;22:1402-10. [Crossref] [PubMed]
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851-60. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Kanda S, Goto K, Shiraishi H, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol 2016;27:2242-50. [Crossref] [PubMed]
- Jotte RM, Socinski MA, Reck M, et al. PS01.53: First-Line Atezolizumab Plus Chemotherapy in Chemotherapy-Naive Patients with Advanced NSCLC: A Phase III Clinical Program: Topic: Medical Oncology. J Thorac Oncol 2016;11:S302-S303. [Crossref] [PubMed]
- Socinski MA, Schiller J, Dakhil S, et al. PS01.54: Evaluation of Novel Blood-Based Biomarkers with Atezolizumab Monotherapy in 1L Advanced or Metastatic NSCLC (B-F1RST): Topic: Medical Oncology. J Thorac Oncol 2016;11:S303-S304. [Crossref] [PubMed]
- Reck M, Papadimitrakopoulou VA, Cappuzzo F, et al. Phase III clinical trials in chemotherapy-naive patients with advanced NSCLC assessing the combination of atezolizumab and chemotherapy. Ann Oncol 2016;27:1294TiP.
- Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med 2016;8:370ra180. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Hellmann M, Gettinger S, Goldman J, et al. CheckMate 012: safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 2016;34:abstr 3001.
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- De Ruysscher D, Pujol J, Popat S, et al. STIMULI: A randomised open-label phase II trial of consolidation with nivolumab and ipilimumab in limited-stage SCLC after standard of care chemo-radiotherapy conducted by ETOP and IFCT. Ann Oncol 2016;27:1430TiP.
- Gerber DE, Urbanic JJ, Langer C, et al. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer 2017;18:333-9. [Crossref] [PubMed]
- Suarez ER. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 2016;7:34341-55. [PubMed]
- Bremnes RM, Busund LT, Kilvær TL, et al. The role of tumor infiltrating lymphocytes in development, progression and prognosis of non-small cell lung cancer. J Thorac Oncol 2016;11:789-800. [Crossref] [PubMed]
- Matsushita H, Kakimi K. γδ T Cell-Based Cancer Immunotherapy. Springer Japan 2016. [Epub ahead of print].
- Baggio L, Laureano AM, Silla LM, et al. Natural killer cell adoptive immunotherapy: Coming of age. Clin Immunol 2016. [Epub ahead of print]. [PubMed]
- Oelsner S, Wagner J, Friede ME, et al. Genetically modified cytokine-induced killer (CIK) cells for targeted cancer therapy. Cancer Immunol Res 2016;4:abstr A164.
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003;9:279-86. [Crossref] [PubMed]
- Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426-35. [Crossref] [PubMed]
- Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13:273-90. [Crossref] [PubMed]
- Zhao Z, van der Stegen SJ, Condomines M, et al. Optimal Activation of Both CD28 and 4-1BB in CAR-Targeted T Cells Results in Enhanced Tumor Eradication. Molecular Therapy 2015;23:S82. [Crossref]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [Crossref] [PubMed]
- Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676-84. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 2012;1:458-66. [Crossref] [PubMed]
- Song DG, Powell DJ. Pro-survival signaling via CD27 costimulation drives effective CAR T cell therapy. Oncoimmunology 2012;1:547-9. [Crossref] [PubMed]
- Guedan S, Carpenito C, Mcgettigan S, et al. Redirection of T(H)17 cells with an ICOS-based CAR enhances function, antitumor activity and persistence of T(H)17 cells. Human Gene Therapy 2012. [Epub ahead of print].
- Tran E, Robbins PF, Lu YC, et al. T cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375:2255-62. [Crossref] [PubMed]
- Tyrakis PA, Palazon A, Macias D, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 2016;540:236-41. [Crossref] [PubMed]
- Roybal KT, Rupp LJ, Morsut L, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016;164:770-9. [Crossref] [PubMed]
- Morsut L, Roybal KT, Xiong X, et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016;164:780-91. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America 1989;86:10024-8. [Crossref] [PubMed]
- Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev 2016;30:157-67. [Crossref] [PubMed]
- Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016;16:566-81. [Crossref] [PubMed]
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015;21:914-21. [Crossref] [PubMed]
- Bonifant CL, Jackson HJ, Brentjens RJ, et al. Toxicity and management in CAR T cell therapy. Mol Ther Oncolytics 2016;3:16011. [Crossref] [PubMed]
- Davies DM, Maher J. Gated chimeric antigen receptor T-cells: the next logical step in reducing toxicity? Transl Cancer Res 2016;5:S61-5. [Crossref]
- Alonso-Camino V, Harwood SL, Alvarez-Mendez A, et al. Efficacy and toxicity management of CAR-T cell immunotherapy: a matter of responsiveness control or tumour-specificity? Biochem Soc Trans 2016;44:406-11. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321-30. [Crossref] [PubMed]
- Huang Y, Wang Y, Duan S, et al. Chimeric Antigen Receptor T Cells in Tumor Immunology: Opportunities and Challenges. J Nanosci Nanotechnol 2016;16:12071-85. [Crossref]
- Geyer MB, Brentjens RJ. Review: Current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy 2016;18:1393-409. [Crossref] [PubMed]
- Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2016;14:802-8. [PubMed]
- Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T cell therapy. Blood 2016;127:2406-10. [Crossref] [PubMed]
- Singh N, Frey NV, Grupp SA, et al. CAR T Cell Therapy in Acute Lymphoblastic Leukemia and Potential for Chronic Lymphocytic Leukemia. Curr Treat Options Oncol 2016;17:28. [Crossref] [PubMed]
- Killock D. Immunotherapy: CAR T cells pursue CLL cells and avoid innocent bystanders. Nat Rev Clin Oncol 2016;13:590-1. [PubMed]
- Atanackovic D, Radhakrishnan SV, Bhardwaj N, et al. Chimeric Antigen Receptor (CAR) therapy for multiple myeloma. Br J Haematol 2016;172:685-98. [Crossref] [PubMed]
- Wang X, Popplewell LL, Wagner JR, et al. Phase 1 studies of central memory-derived CD19 CAR T cell therapy following autologous HSCT in patients with B-cell NHL. Blood 2016;127:2980-90. [Crossref] [PubMed]
- Newick K, Moon E, Albelda SM. Chimeric antigen receptor T cell therapy for solid tumors. Mol Ther Oncolytics 2016;3:16006. [Crossref] [PubMed]
- Junghans RP, Ma Q, Rathore R, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016;76:1257-70. [Crossref] [PubMed]
- Emami-Shahri N, Foster J, Sosabowski J, et al. Abstract 2315: Dynamic SPECT imaging of PSMA-specific CAR T cells in mice bearing prostate cancer. Cancer Res 2016;76:2315. [Crossref]
- Liu W, Han W, Zhou L, et al. Mp84-15 Prevent Metastasis by Double Chimeric Antigen Receptor T Cells Targeting Circulating Cancer Cells for Prostate Cancer Treatment. J Urol 2016;195:e1095. [Crossref]
- Alrifai D, Sarker D, Maher J. Prospects for adoptive immunotherapy of pancreatic cancer using chimeric antigen receptor-engineered T cells. Immunopharmacol Immunotoxicol 2016;38:50-60. [Crossref] [PubMed]
- Mohammed S, Sukumaran S, Bajgain P, et al. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol Ther 2017;25:249-58. [Crossref] [PubMed]
- Mayor M, Zeltsman M, McGee E, et al. A regional approach for CAR T cell therapy for mesothelioma: from mouse models to clinical trial. Immunotherapy 2016;8:491-4. [Crossref] [PubMed]
- Krenciute G, Balyasnikova I, Dotti G, et al. 76. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells. Mol Ther 2016;24:S33. [Crossref]
- Künkele A, Taraseviciute A, Finn LS, et al. Preclinical Assessment of CD171-Directed CAR T cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin Cancer Res 2017;23:466-77. [Crossref] [PubMed]
- Harrer D, Simon B, Schuler G, et al. 173 γ/δ T cells RNA-transfected with a T cell receptor and a chimeric antigen receptor specific for melanoma. J Invest Dermatol 2016;136:S190. [Crossref]
- Kailayangiri S, Altvater B, Spurny C, et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. OncoImmunology 2016.e1250050. [PubMed]
- Ruella M, Levine BL. Smart CARS: optimized development of a chimeric antigen receptor (CAR) T cell targeting epidermal growth factor receptor variant III (EGFRvIII) for glioblastoma. Ann Transl Med 2016;4:13. [PubMed]
- Vora P, Venugopal C, Mahendram S, et al. Abstract 2300: Human CD133-specific chimeric antigen receptor (CAR) modified T cells target patient-derived glioblastoma brain tumors. Cancer Res 2016;76:2300. [Crossref]
- Cogdill AP, Boesteanu A, Xu C, et al. Toxicity testing of EGFRvIII CAR-based immunotherapy of glioblastoma: From bench to bedside. Cancer Immunol Res 2016;4:abstr B139.
- Vora P, Chokshi C, Qazi M, et al. The efficacy of CD133 BiTEs and CAR-T cells in preclinical model of recurrent glioblastoma. Cancer Immunol Res 2016;4:abstr B079.
- Shiina S, Ohno M, Ohka F, et al. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer Immunol Res 2016;4:259-68. [Crossref] [PubMed]
- Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T cells forward. Nat Rev Clin Oncol 2016;13:370-83. [Crossref] [PubMed]
- Sandfeld-Paulsen B, Aggerholm-Pedersen N, Baek R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol 2016;10:1595-602. [Crossref] [PubMed]
- You F, Jiang L, Zhang B, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci 2016;59:386-97. [Crossref] [PubMed]
- Maher J, Wilkie S, Davies DM, et al. Targeting of Tumor-Associated Glycoforms of MUC1 with CAR T Cells. Immunity 2016;45:945-6. [Crossref] [PubMed]
- Posey AD Jr, Schwab RD, Boesteanu AC, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016;44:1444-54. [Crossref] [PubMed]
- Han W, Du Y. Recent Development of the Second and Third Generation Irreversible EGFR Inhibitors. Chem Biodivers 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Zhou X, Li J, Wang Z, et al. Cellular Immunotherapy for Carcinoma Using Genetically Modified EGFR-Specific T Lymphocytes. Neoplasia 2013;15:544-53. [Crossref] [PubMed]
- Feng K, Guo Y, Dai H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci 2016;59:468-79. [Crossref] [PubMed]
- Ahmed N, Brawley VS, Hegde M, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol 2015;33:1688-96. [Crossref] [PubMed]
- Zhao Q, Lui S, Li N, et al. The Application and Mechanism of Costimulation-Enhanced Chimeric Antigen Receptor-T Cells in the Treatment of Lung Cancer. Cytotherapy 2016;18:S96. [Crossref]
- Pilia G, Hughes-Benzie RM, MacKenzie A, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 1996;12:241-7. [Crossref] [PubMed]
- DeBaun MR, Ess J, Saunders S. Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition. Mol Genet Metab 2001;72:279-86. [Crossref] [PubMed]
- Li W, Guo L, Rathi P, et al. Redirecting T Cells to Glypican-3 with 4-1BB Zeta Chimeric Antigen Receptors Results in Th1 Polarization and Potent Antitumor Activity. Hum Gene Ther 2017;28:437-48. [PubMed]
- Wu Y, Liu H, Ding H. GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocell Carcinoma 2016;3:63-7. [Crossref] [PubMed]
- Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20:6418-28. [Crossref] [PubMed]
- Li K, Pan X, Bi Y, et al. Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget 2016;7:2496-507. [PubMed]
- Rettig WJ, Garin-Chesa P, Beresford HR, et al. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A 1988;85:3110-4. [Crossref] [PubMed]
- Vicent S, Sayles LC, Vaka D, et al. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Res 2012;72:5744-56. [Crossref] [PubMed]
- Kakarla S, Chow KK, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 2013;21:1611-20. [Crossref] [PubMed]
- Kinch MS, Moore MB, Harpole DH Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res 2003;9:613-8. [PubMed]
- Amato KR, Wang S, Tan L, et al. EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res 2016;76:305-18. [Crossref] [PubMed]
- Iwahori K, Kakarla S, Velasquez MP, et al. Engager T cells: a new class of antigen-specific T cells that redirect bystander T cells. Mol Ther 2015;23:171-8. [Crossref] [PubMed]
Cite this article as: Wu Y, Jiang M. The revolution of lung cancer treatment: from vaccines, to immune checkpoint inhibitors, to chimeric antigen receptor T therapy. Biotarget 2017;1:7.