
Uronic acid pathway metabolites regulate mesenchymal transition and invasiveness in lung adenocarcinoma
Metastatic transition results in the greatest morbidity and mortality in patients with cancer (1). Metastasis is a step-wise process that results in local extravasation and hematological dissemination of epithelial tumors (1). Local extravasation involves remodeling extracellular matrix (ECM) coupled with cellular invasion, a process promoted by epithelial mesenchymal transition (EMT). EMT is a complex cellular reprogramming event resulting in dissolution of mucosal tight junctions, loss of apical-basal polarity, reorganization of the cytoskeleton promoting motility and secretion of ECM-modifying proteins (2,3). In epithelial tumors, EMT is triggered by its local stromal microenvironment, including inflammatory cytokines, presence of hypoxia and secretion of epithelial growth factors. Understanding the factors controlling EMT in stem-ness, metastatic potential, chemotherapy resistance and how EMT can be modulated to affect cancer metastasis have been the focus of intense investigation.
Divided into two distinct classes of non-small carcinoma (adenocarcinoma) and small cell carcinomas, lung cancer is the leading cause of cancer deaths worldwide (4). Cell transformation in non-small cell carcinoma is driven by the KRAS oncogene; found in up to 25% of lung adenocarcinomas. A hallmark of transformation is metabolic reprogramming whose presence was initially discovered by the finding that cancer cells have enhanced aerobic glycolysis [known as the Warburg effect (5)]. More recently, metabolic studies of Ras-oncogene driven cancers have shown increased glucose flux and glucosamine metabolic activity directing enhanced nutrient acquisition (6). Additionally, cancer cells are metabolically “adaptive”, meaning they are able to respond, survive and grow in nutrient poor and/or hypoxic conditions, providing survival advantage. Metabolic reprogramming in metastatic cancers disease has been thought to be a secondary adaptation produced by selective pressure. New evidence indicates that reprograming of glucose metabolism occurs at several levels (Figure 1). Recent work has shown that EMT activates the hexosamine biosynthetic pathway (HBP) and elevates the level of UDP-N acetylglucosamine, an intermediate required for N glycosylation, folding and secretion of ECM proteins to maintain proteostasis. Experiments inhibiting the rate-limiting enzyme of HBP indicate that its activity is required for maintaining EMT (7). However, the relationship between EMT-induced metabolic reprogramming and cellular motility has not been fully understood. Activation of HBP in EMT also induces an aberrant cell surface glycosylation and O-GlcNAcylation and modulates cell plasticity (8).
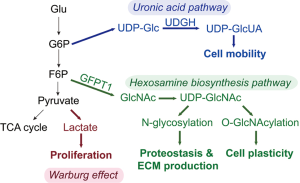
Using a pathway-focused siRNA-mediated knockdown survey of 111 rate-limiting metabolic proteins in KRAS-mutated A549 lung adenocarcinoma cells, Wang et al. discovered that knockdown of UDP-glucose 6-dehydrogenase (UGDH) inhibits cellular motility (9). UGDH is a single-copy NAD+-linked oxidoreductase that is the rate-limiting enzyme in formation of UDP-glucuronic acid (UDP-GlcUA), important in hyaluronan and glycosaminoglycan (GAG) production. In repletion experiments of UGDH-silenced cells, UDP-GlcUA was found to restore hyaluronan and GAG production, but not cellular motility, separating downstream cell migration from that of GAG synthesis. EMT is multi-step cell-state transition (10) whose terminal maintenance is controlled by a SNAI1-ZEB autoregulatory feedback loop (11). Wang et al. examined the abundance of SNAI1 in UGDH depleted cells and discovered that SNAI1 expression was substantially reduced. UDP-GlcUA repletion did not enhance the expression of SNAI, but ectopic SNAI expression restored migration in UGDH-depleted cells, indicating that the loss of migration was mediated through SNAI1 and/or its downstream regulatory pathway.
SNAI1 depletion in UGDH-silenced cells was not from decreased SNAI1 transcription, but rather reduced SNAI1 mRNA stability. Wang et al. examined the effect of epidermal growth factor (EGF) stimulation on SNAI1 and found that the EGF-triggered induction of SNAI1 mRNA stability was not observed in UGDH-depleted cells. In a series of experiments involving affinity-mass spectrometry profiling, UGDH was found to associate with ELAV1/HuR. ELAV1/HuR is an RNA-binding protein recognizing adenylate uridylate rich elements (AREs) in the 3'UTR, whose binding stabilizes mRNAs important in embryonic stem cell differentiation, including MMP9 and TGFβ1 mRNAs (Figure 2). Mutagenesis of the three AREs found in the SNAI1 mRNA 3'UTR resulted in the loss of ELAV1/Hur binding and changes in SNAI1 mRNA stability. Wang et al. demonstrate that the UGDH-ELAV1/Hur interaction was EGF-dependent, and the interaction was enriched in the nucleus. Although EGF stimulation induced ELAV1/Hur phosphorylation and this phosphorylation was required for UGDH-ELAV1/Hur interaction, an oxidoreductase mutation of UGDH was unable to confer SNAI1 stability. Interestingly, the ELVA1/Hur RNA binding domain bound both the precursor metabolite, UDP-Glc, and the enzymatic product, UDP-GlcUA, of UGDH. The binding of each metabolite occurs in apparently two different modes involving distinct side-chain interactions. Because of these different modes of binding, UDP-GLc is a more potent inhibitor of ELAV1/Hur-SNAI1 ARE binding.
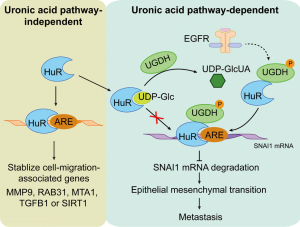
In a potentially translational application, metastatic tumors were observed to have lower levels of UDP-GLc than primary matched tumors. Moreover, supplementation of UDP-Glc functions in a tumor suppressor role inhibiting cellular motility in KRAS-driven A549 adenocarcinoma cells. Additional experiments demonstrating the UGDH interaction with ELAV1/Hur is phosphorylation-dependent and not bridged by SNAI1 RNA were presented. The UGDH tyrosine phosphoacceptor site, Tyr-473, was demonstrated to be EGF inducible in a mitogen activated protein kinase-dependent manner. This phosphorylation was functionally important because a Tyr-Glu [473] site mutation of UGDH blocked its binding to ELVA1/Hur and prevented SNAI1 mRNA stabilization (Figure 2). Clinical correlation was established examining the abundance of phospho-Y473 UGDH and SNAIL expression in 114 lung cancer specimens. The levels of phospho-Y473 UGDH and SNAIL protein were strongly correlated. Patients with high levels of phospho-Y473 UGDH had a detectably lower median survival in response to standard of care (radio- and chemotherapy post-surgery).
These exciting, elegant and compelling studies demonstrate an important relationship between UGDH, mesenchymal transition and cellular motility in the metastatic transition of lung adenocarcinoma. There are several important implications of this work in EMT biology and translational implications.
The results of this study indicate that the cellular motility response in EMT is downstream of SNAI1. SNAI is a C2H2 zinc finger-containing transcription factor that functions in normal development as a mesodermal determinant. SNAI1 can function either as an activator or as a repressor depending on promoter context and cell-specific expression of repressor complexes. Ectopic expression of SNAI in breast cancer cells induces mesenchymal transition by inducing some 400 genes, and silencing some 300 genes. However, these data should be interpreted with the understanding that protein complexes formed and gene targets of many mesenchymal regulators are highly cell-type specific (12). Genome-wide CHiP-Seq studies have identified that a major activity of SNAI is to repress cellular adhesion genes found in differentiated epithelial cells (ECDH, claudins) (13). SNAI1-mediated gene silencing is mediated by interactions of repressor proteins [histone deacetylases (HDACs), DNA methylases] with its SNAG (Snail/Slug, and Gfi-1) NH2 terminal domain (14). SNAI1 also activated mesenchymal gene expression programs by recruiting activator complexes. Through this activity, ectopic SNAI1 expression induces cellular motility and promotes an anti-apoptotic state. The mechanisms for induction of cell motility have not been fully explored; direct targets of SNAI1 activation include small molecule GTPases (13), important regulators of cytoskeletal contraction, or the effect may be indirect. More work will need to be done to understand the promoter-constrained factors that determine whether SNAI1 functions as an activator or repressor, and what downstream targets control cellular motility.
The effect of Ras-mediated oncogenic transformation on metabolic pathways includes enhanced flux of glucose and glutamine metabolism. Ras enhances glucose uptake via enhanced expression of glucose transporter 1 (GLUT1) and utilization by anabolic pathways (6). Ras also directs glutamine carbon into biosynthetic, redox homeostasis and cell growth pathways (15). On top of this background, we now recognize complex metabolic adaptations occur in the process of EMT. Importantly, these metabolic states are not simply compensatory, but contribute directly to distinct functions seen in the transition. The study of Wang et al. indicate EGF induces upregulation of rate-limiting proteins involved in UDP-GlcUA formation, forming metabolic products that have allosteric effects on RNA stability. We also have applied systems-level perturbations to discover that EMT is also triggers the HBP downstream of the unfolded protein response (UPR). This process UDP-N-acetylglucosamine to enhance N glycosylation of fibronectin (FN), promoting its post-translational folding in the endoplasmic reticulum (ER) and secretion to maintain proteostasis. Experiments inhibiting the rate-limiting enzyme of HBP indicate that its activity is required for maintaining EMT (7). Rate-limiting proteins involved in GlcNAc formation are induced, altering protein glycosylation. For example, FN is one of the most highly upregulated proteins in TGFβ-stimulated lung epithelial cells entering EMT (7). Alternatively, glycated FN interacts with integrin subunits, directing intracellular pro-survival signaling. Interestingly, GlcNAc is also a post-translational modification that competes with intracellular phosphorylation. For example, GlcNAc modifies phosphorylation of the master EMT regulator, RelA, and the signaling pathways under its control (16). In this way, the HBP and its effects maintain proteostasis are required for sustained adaptation to the mesenchymal transition (7).
The complex transition of the EMT involves multiple intermediate states involving transcriptional factor feedback and feed-forward pathways producing “partial EMT states” (10). Our understanding of these partial EMT states in clinical disease is rudimentary. The stable EMT phenotype has been studied through integrated systems approaches (16-18). We know that the induction of the core mesenchymal transcription factors, SNAI1-ZEB are coordinately controlled at the level of transcription, RNA stability, and protein stabilization. In response to TGFβ stimulation, SNAI1 and ZEB genes are transcriptionally activated by the RelA master regulator through a process of transcriptional elongation involving the bromodomain containing protein 4 (BRD4) (19) Perturbation of the SNA1 protein disrupts a microRNA autoregulatory feedback loop that normally silences SNAI1-ZEB (11). The study of Wang et al. provides additional information that intracellular UDP-Glc is an important allosteric modulator of ELAV1/Hur, affecting SNAI1 mRNA steady-state abundance. In concert, the SNAI1 protein is also controlled by exogenous cytokine stimulation that promotes additional transcription and protein stabilization by RelA (20). This complex and coordinate regulation results in highly inducible SNAI1 expression through interactions with the stromal microenvironment.
There is a substantial effect of the inflammatory stromal environment on the mesenchymal transition and metastatic behavior of epithelial carcinomas (21). Factors released by cancer-associated fibroblasts include IL-6 cytokines signaling through STAT, upregulating TGFβ and promoting chemotherapy resistance. Of relevance here ELAV1/Hur expression is inducible by cytokines, perhaps providing one the mechanisms by which an inflammatory stromal microenvironment triggers EMT.
Wang et al. provide evidence that enhanced expression of pELAV1/Hur and SNAI1 cellular markers of mesenchymal transition are associated with reduced survival in patients with conventional treatment. These findings are consistent with earlier studies that have identified more classical markers of mesenchymal transition as markers for reduced survival (22) and resistance to EGFR inhibitor therapy (23), including VIM, SNAI1, ECDH and others in human non-small cell cancers. The explanations for reduced survival in patients with EMT signature positive cancers may be related to enhanced motility and chemotherapy resistance characteristic of the mesenchymal state. However, it is important to keep in perspective that EMT induces resistance to DNA damage, triggers stem-cell like behaviors (24) and affects immune-modulatory activities (25). The relative roles of enhanced motility, apoptosis resistance, stem cell-like properties and immunomodulation have yet to be fully understood.
Finally, the study by Wang suggests that targeting the uronic pathway may hold promise in the reduction of metastatic progression in lung adenocarcinoma. This elegant mechanistic elucidation should be interpreted with the perspective that unfortunately, most lung cancers are diagnosed after metastatic dissemination has already occurred. Advancing treatment of lung cancers by modifying the uronic pathway will need to be paired with interventions that identify high risk patients with primary adenocarcinoma before metastatic disease has occurred.
Acknowledgments
Funding: This work was funded in part through NIH 1R21 AI133454 (Y Zhao, AR Brasier).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [Crossref] [PubMed]
- Ijaz T, Pazdrak K, Kalita M, et al. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J 2014;7:13. [Crossref] [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [Crossref] [PubMed]
- Román M, Baraibar I, López I, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer 2018;17:33. [Crossref] [PubMed]
- Warburg O. On respiratory impairment in cancer cells. Science 1956;124:269-70. [PubMed]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 2015;17:351-9. [Crossref] [PubMed]
- Zhang J, Jamaluddin M, Zhang Y, et al. Type II Epithelial-Mesenchymal Transition Upregulates Protein N-Glycosylation To Maintain Proteostasis and Extracellular Matrix Production. J Proteome Res 2019;18:3447-60. [Crossref] [PubMed]
- Lucena MC, Carvalho-Cruz P, Donadio JL, et al. Epithelial Mesenchymal Transition Induces Aberrant Glycosylation through Hexosamine Biosynthetic Pathway Activation. J Biol Chem 2016;291:12917-29. [Crossref] [PubMed]
- Wang X, Liu R, Zhu W, et al. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature 2019;571:127-31. [Crossref] [PubMed]
- Zhang J, Tian XJ, Zhang H, et al. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal 2014;7:ra91. [Crossref] [PubMed]
- Lu M, Jolly MK, Levine H, et al. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci U S A 2013;110:18144-9. [Crossref] [PubMed]
- Chang H, Liu Y, Xue M, et al. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res 2016;44:2514-27. [Crossref] [PubMed]
- Maturi V, Morén A, Enroth S, et al. Genomewide binding of transcription factor Snail1 in triple-negative breast cancer cells. Mol Oncol 2018;12:1153-74. [Crossref] [PubMed]
- Hou Z, Peng H, White DE, et al. 14-3-3 binding sites in the snail protein are essential for snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res 2010;70:4385-93. [Crossref] [PubMed]
- Gaglio D, Metallo CM, Gameiro PA, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 2011;7:523. [Crossref] [PubMed]
- Zhao Y, Tian B, Sadygov RG, et al. Integrative proteomic analysis reveals reprograming tumor necrosis factor signaling in epithelial mesenchymal transition. J Proteomics 2016;148:126-38. [Crossref] [PubMed]
- Tian B, Li X, Kalita M, et al. Analysis of the TGFbeta-induced program in primary airway epithelial cells shows essential role of NF-kappaB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genomics 2015;16:529. [Crossref] [PubMed]
- Tian B, Widen SG, Yang J, et al. The NFkappaB subunit RELA is a master transcriptional regulator of the committed epithelial-mesenchymal transition in airway epithelial cells. J Biol Chem 2018;293:16528-45. [Crossref] [PubMed]
- Tian B, Zhao Y, Sun H, et al. BRD4 Mediates NFkB-dependent Epithelial-Mesenchymal Transition and Pulmonary Fibrosis via Transcriptional Elongation. Am J Physiol Lung Cell Mol Physiol 2016;311:L1183-201. [Crossref] [PubMed]
- Wu Y, Deng J, Rychahou PG, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 2009;15:416-28. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Gordian E, Welsh EA, Gimbrone N, et al. Transforming growth factor β-induced epithelial-to-mesenchymal signature predicts metastasis-free survival in non-small cell lung cancer. Oncotarget 2019;10:810-24. [Crossref] [PubMed]
- Byers LA, Diao L, Wang J, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 2013;19:279-90. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Ricciardi M, Zanotto M, Malpeli G, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer 2015;112:1067-75. [Crossref] [PubMed]
Cite this article as: Zhao Y, Brasier AR. Uronic acid pathway metabolites regulate mesenchymal transition and invasiveness in lung adenocarcinoma. Biotarget 2019;3:19.


