
A multi-layer post-transcriptional gene regulatory program fuels cancer angiogenesis and metastasis
Introduction
Metastasis is the process by which cancer cells spread from the primary tumor to surrounding tissues and to distant organs. It is the primary cause of cancer morbidity and mortality, and represent a major clinical problem, where it is estimated to account for ~90% of cancer deaths (1). It has been suggested that metastasis can be represented as a biphasic process, starting with the physical translocation of cancer cells to a distant organ, followed by their capacity to spread, invade, and thrive in non-native environments (1). Several hypotheses have been proposed to describe the cellular processes involved in cancer metastasis such as epithelial mesenchymal transition, accumulation of mutations, macrophage facilitation process, and macrophage transformation or fusion hybridization with neoplastic cells. However, metastasis process remains one of the most inexplicable aspects of cancer at the molecular level (1). Therefore, finding the right molecular targets for therapeutic intervention is crucial for prevention and treatment success.
Among the different processes involved in metastasis, angiogenesis is regarded as a key component. Besides its role in nourishing the growing tumour mass, angiogenesis can also perform a dual role in cancer metastasis: it can help tumor cells escape into the bloodstream, and it is required for establishment of metastatic colonies in secondary sites. Most of the currently used anti-angiogenic therapeutic strategies focus on inhibition of angiogenic factors such as members of the fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) families. However, the therapeutic effects of these agents are modest and prone to numerous resistance mechanisms that are often followed by restoration of tumor growth and progression (2). Of importance, anti-angiogenic drugs targeting physiologically important molecules, such as VEGFA, can cause serious and potentially lethal side effects due to their pleiotropic role in multiple organs (3). This raises the importance of identifying key drivers behind angiogenesis, which could be targets for more specific, efficient and preventive treatments of metastasis.
Azam et al. recently identified miR-200 family as a key player in cancer metastasis, shedding new light on the direct and indirect mechanisms by which this miRNA regulates different aspects of cancer angiogenesis and metastasis (4). Here, we review the important roles and the molecular mechanisms through which the miR-200 family operates in cancer. We particularly focus on the recent study by Azam et al. that deciphers a miR-200-mediated post-transcriptional gene regulatory program that controls angiogenesis and metastasis, and the implications of their findings in development of potential therapeutic strategies.
MicroRNAs (miRNAs) and cancer
With ~2,500 members, miRNAs represent the largest class of the factors that regulate gene expression post-transcriptionally. Deregulation of miRNAs has been broadly reported in several cancers and is correlated with cancer type, stage, and other clinical variables (5). The miR-200 family members are among the most well studied miRNAs in cancer, and have been shown to play vital roles in cancer angiogenesis and metastasis (6). The miR-200 family consists of five members divided into two groups that differ by 1 nucleotide in their seed sequences, therefore having both overlapping and non-overlapping targets. One group consists of miR-200a and miR-141, whereas miR-200b, miR-200c, and miR-429 constitute the other group.
miR-200 expression is associated with clinical outcome
The miR-200 family has been reported to affect every step of the sequential events that lead to metastasis, including angiogenesis, intravasation, survival in circulation, extravasation, and secondary tumor formation (6). The exact role of miR-200 family members, however, appears to be dependent on the cancer type. For example, while miR-200 over-expression can inhibit the formation of distant metastasis in lung cancer, it has been reported to enhance metastasis in breast cancer models (7,8). In fact, higher miR-200 was reported to be associated with better clinical outcome in ovarian, lung and renal cancers, while it is associated with worse overall survival in basal-like breast adenocarcinomas (7). This heterogeneity can be even observed at the level of cancer subtypes, where the poor survival associated with high miR-200 expression in breast cancer was only significant for the luminal subtypes, which was related to the unique gene networks regulated by miR-200 in each subtype (7). In this regard, miR-200 was shown to regulate DNA-dependent transcription and metabolic processes in luminal type, whereas it regulates angiogenic networks in basal-like breast cancers (7). Interestingly, similar associations with angiogenic pathways were identified in high-grade ovarian cancers, renal and lung adenocarcinomas. Of importance, IL-8, a target of miR-200 that is involved in angiogenesis and metastasis, was found to have the opposite association with clinical outcomes compared to miR-200 (7). Altogether, these data further support the association of miR-200 with clinical outcomes, which could be related to its role in angiogenesis.
miR-200 is a master regulator of tumor angiogenesis
Since its discovery as a master regulator of epithelial-mesenchymal transition (EMT), the miR-200 family has been shown to regulate the complex network of angiogenesis-related genes and pathways (Figure 1). Downregulation of miR-200 family members is a vital step for tumor cells to undergo EMT, invade, and metastasize. Likewise, overexpression of miR-200 was able to recreate the transcriptional features of metastasis-incompetent tumor cells in a syngeneic lung adenocarcinoma metastasis model. This has been associated with the regulation of several key factors in tumor angiogenesis including E-cadherin, IL-8, CXCL1, VEGF and WAVE3 (Figure 1). By targeting E-cadherin transcriptional repressors ZEB1 and ZEB2, miR-200b indirectly enhances E-cadherin expression and, therefore, inhibits EMT and cancer cell migration (9). MiR-200 can also inhibit angiogenesis through direct targeting of interleukin-8 and CXCL1 in tumour endothelial and cancer cells (7). IL-8 and CXCL1 are known to exert their pro-angiogenic effects in an autocrine fashion through endothelial CXCR2 receptors. Importantly, three independent studies have shown that miR-200b and miR-200c can inhibit lung adenocarcinoma cell invasion by directly targeting VEGF and its receptors (10,11), which are required for cell invasion and metastasis, and have been associated with worse outcomes in adenocarcinoma (11) and clear cell renal cell carcinoma (12). On the other hand, miR-200 can regulate the motility, migration and invasion of transformed cells by inhibiting regulators of the actin cytoskeleton such as WAVE3, FHOD1 and PPM1F (13,14). Interestingly, conditioned media from arsenic-transformed cells stably expressing miR-200b were less potent to induce HUVECs tube formation in vitro, which suggests the involvement of miR-200 in the cross-talk between tumour cells and their microenvironment (15). In this regard, miR-200 was shown to regulate breast cancer cell secretome by inhibiting Sec23a expression (8). Interestingly, while ectopic miR-200 expression reduced early cancer cell dissemination in this study of breast cancer, it enhanced colonization step in distant organs, suggesting a dichotomous biphasic role for miR-200 during metastasis.
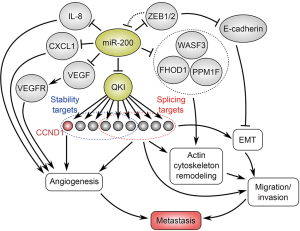
In their recent report, Azam et al. identified a novel indirect mechanism by which miR-200 can regulate endothelial cell (EC) sprouting and microvessel formation, by inhibiting CCND1 through targeting the RNA binding protein (RBP) Quaking (QKI) (4). QKI was reported to stabilize CCND1 mRNA and to be a main target of miR-200 family (Figure 1). Up-regulation of CCND1, a regulatory subunit of CDK4/CDK6 that is required for cell cycle G1/S transition, has been associated with poor outcomes in a variety of cancers (16). QKI targeting was found to be the main mechanism through which miR-200 regulated EC sprouting as shown both in vitro and in vivo (4). Similarly, CCND1 inhibition recapitulated the effects of QKI silencing on EC function in vitro, and on tumor angiogenesis and metastasis in vivo, which highlights the importance and therapeutic potential of the miR-200b/QKI/CCND1 axis in regulating tumor angiogenesis and metastasis (4). However, this is in contrast to previous reports describing QKI as a tumor suppressor (17,18), which could be related to its specific spatiotemporal expression in tumor microenvironment. Indeed, both QKI and CCND1 expression and their interaction is dependent on EC cell cycle progression (4,16). In addition, QKI siRNA delivery to tumor endothelium did not inhibit primary tumor growth, which points to the cell and context-specific effects of this RBP on tumor progression. Besides, the pleiotropic role of QKI in the regulation of a large arsenal of gene networks may also contribute to cancer progression through other mechanisms (18-20). Interestingly, miR-200 was shown to regulate hundreds of genes involved in cell migration and invasion, including several actin cytoskeleton-associated genes, through QKI-mediated alternative splicing, which further underscores the role of QKI in mediating miR-200 function in cancer cells (19) (Figure 1). This novel and interesting mechanism by which miR-200 indirectly regulates a large number of genes through modulation of an RBP underlines the importance of miRNAs as master regulators of gene expression and their potential as therapeutic targets.
In vivo delivery and restoration of miR-200 function
Delivery of miRNAs to target tissues, with the aim of restoring their in vivo function, remains challenging due to physiological barriers that prevent cellular uptake, as well as degradation by nucleases (21). Therefore, altering the biochemical properties or using synthetic carrier molecules is required to delay plasma clearance and promote tissue and cellular penetrance of these molecules for in vivo delivery. Polymer-based carriers, such as chitosan, polyethyleneimine and polyamidoamine dendrimers, have been advantageous as carriers for effective delivery systems of miRNA mimics and inhibitors (21). Synthetic “miRNA mimics”, double-stranded RNAs that can be used to replace a downregulated miRNA, are smaller and more stable than mRNAs, allowing ease of encapsulation in several types of carrier polymers.
In their study, Azam et al. successfully delivered miR-200 oligonucleotides into all cancerous tissues, including metastatic lesions in the spleen and liver, using 2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomal nanoparticles through both intravenous and intraperitoneal routes. This resulted in reduction in the primary tumour burden and proliferation, as well as reduction in angiogenesis and distant metastatic aggregates (7). To assess the efficiency of therapeutic miR-200 delivery, the authors measured validated miR-200 targets such as ZEB1, QKI and IL-8 (7). DOPC liposomes are characterized by a neutral charge, which enables them to deliver cargo molecules beyond tumor vasculature and into cancer cells. This feature makes DOPC liposomal nanoparticles an effective miRNA delivery vehicle, having been successfully used to induce marked anti-angiogenic and therapeutic effects in different cancer models (4,7,21).
Despite their efficiency in delivering RNAi molecules to orthotopic tumors, neutral nanoliposomal carriers allow limited delivery of siRNA into the tumor-associated ECs (21). In fact, new approaches are being developed for intentional targeting of tumor microenvironment, which has been shown to play vital roles in tumor progression. Chitosan-based nanoparticle is an attractive option for this purpose due to low immunogenicity and low toxicity, as well as its chemical features that allow for chemical conjugation of specific ligands for targeted therapy (21). For example, Arg-Gly-Asp (RGD)-labelled chitosan nanoparticles can bind to αvβ3 integrin, a marker of angiogenic vasculature that could be targeted to specifically induce apoptosis of proliferative angiogenic vascular cells (22). Azam et al. (5) and others (4) suggest that miR-200 therapeutic effects are prominent after targeted delivery to the endothelium using chitosan nanoparticles (4,7). Particularly, the combination of miR-200a and -200b led to a much higher reduction in tumor volume and angiogenesis compared to either one alone, which may point to the efficacy and specificity of this approach (7). In the study by Azam et al., apart from miRNA delivery, QKI silencing through siRNA delivery to ECs was also able to reduce distant cancer metastasis, which raises the possibility of using this approach for directed gene therapy (4).
Clinical perspectives
A growing line of evidence points to the critical role of EMT and tumor angiogenesis in tumor invasion and metastasis. Recent studies support the concept of evasive resistance to therapy, wherein the tumor cells can evade the specific targets of therapy through alternative molecular pathways that can compensate for the loss of function induced by the treatment (2). This mode of resistance has been particularly demonstrated for the currently used anti-angiogenic drugs.
MicroRNAs are master regulators of gene expression and have the capacity to target hundreds of genes simultaneously, which makes them attractive candidates as prognostic biomarkers and therapeutic targets in cancer. The extensive role of miR-200 in the regulation of different oncogenic processes and its key role in the regulation of angiogenesis and metastasis underscores the biological and therapeutic implications of this miRNA family for cancer treatment. Since the miR-200 family functions as putative tumor suppressors and its down-regulation is associated with poor outcome in various cancers, restoration of miR-200 expression may have therapeutic implications for the treatment of metastatic and drug-resistant tumors.
Azam et al. have shown that the effects of miR-200 can go far beyond their direct targets through the regulation of other regulators of gene expression such as RBPs. The latter are prime factors that regulate post-transcriptional gene regulatory programs through RNA processing, stability, and localization, among their other functions. Several studies have shown that deregulation of RBPs results in aberrations of cancer-associated pathways through driving abnormal post-transcriptional gene regulatory programs (4,17-20,23). The paper by Azam et al. sheds new light on the complexity of mechanisms that underlie abnormal gene expression in cancer at the post-transcriptional level by showing that miRNAs and RBPs can mediate and perhaps potentiate regulatory effects of each other. It is important to note that while the study by Azam et al. primarily focused on the role of a miRNA in regulating an RBP, RBPs can also in turn affect miRNA function through modulation of their processing (18,20) or interfering with the binding of miRNAs to specific mRNA targets, thereby inhibiting miRNA function (24).
Suppressing and restoring miRNAs for therapeutic purposes are difficult with conventional therapies. Despite their promising effects, RNA-based treatments are still immature and face substantial challenges including physiological barriers and systemic toxicity of nanoparticles. The emerging interactions between miRNAs and RBPs open new avenues to harness the potentials of miRNAs in cancer treatment through targeting their partner/target RBPs. Experimental and computational methods are being developed to better understand post-transcriptional regulatory networks and interactions of different factors involved in these regulatory programs (23-25). Active research in this field promises the development of a new generation of targets that mainly function as the regulators of post-transcriptional gene expression in cancer.
Acknowledgments
Funding: This work was supported by the Canadian Institutes of Health Research (CIHR) grant PJT-155966 to HS Najafabadi and Y Riazalhosseini. HS Najafabadi holds a CIHR Canada Research Chair in Systems Biology of Gene Regulation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [Crossref] [PubMed]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592-603. [Crossref] [PubMed]
- Stone RL, Sood AK, Coleman RL. Collateral damage: toxic effects of targeted antiangiogenic therapies in ovarian cancer. Lancet Oncol 2010;11:465-75. [Crossref] [PubMed]
- Azam SH, Porrello A, Harrison EB, et al. Quaking orchestrates a post-transcriptional regulatory network of endothelial cell cycle progression critical to angiogenesis and metastasis. Oncogene 2019;38:5191-210. [Crossref] [PubMed]
- Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol 2009;4:199-227. [Crossref] [PubMed]
- Humphries B, Yang C. The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015;6:6472-98. [Crossref] [PubMed]
- Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun 2013;4:2427. [Crossref] [PubMed]
- Korpal M, Ell BJ, Buffa FM, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 2011;17:1101-8. [Crossref] [PubMed]
- Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008;283:14910-4. [Crossref] [PubMed]
- Choi YC, Yoon S, Jeong Y, et al. Regulation of Vascular Endothelial Growth Factor Signaling by miR-200b. Mol Cells 2011;32:77-82. [Crossref] [PubMed]
- Roybal JD, Zang Y, Ahn YH, et al. miR-200 Inhibits Lung Adenocarcinoma Cell Invasion and Metastasis by Targeting Flt1/VEGFR1. Mol Cancer Res 2011;9:25-35. [Crossref] [PubMed]
- Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol 2010;4:51. [Crossref] [PubMed]
- Sossey-Alaoui K, Bialkowska K, Plow EF. The miR200 Family of MicroRNAs Regulates WAVE3-dependent Cancer Cell Invasion. J Biol Chem 2009;284:33019-29. [Crossref] [PubMed]
- Jurmeister S, Baumann M, Balwierz A, et al. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol Cell Biol 2012;32:633-51. [Crossref] [PubMed]
- Wang Z, Humphries B, Xiao H, et al. Epithelial to mesenchymal transition in arsenic-transformed cells promotes angiogenesis through activating β-catenin-vascular endothelial growth factor pathway. Toxicol Appl Pharmacol 2013;271:20-9. [Crossref] [PubMed]
- John RR, Malathi N, Ravindran C, et al. Mini review: Multifaceted role played by cyclin D1 in tumor behavior. Indian J Dent Res 2017;28:187-92. [Crossref] [PubMed]
- Zhou X, Li X, Sun C, et al. Quaking-5 suppresses aggressiveness of lung cancer cells through inhibiting β-catenin signaling pathway. Oncotarget 2017;8:82174-84. [PubMed]
- Chen AJ, Paik JH, Zhang H, et al. STAR RNA-binding protein Quaking suppresses cancer via stabilization of specific miRNA. Genes Dev 2012;26:1459-72. [Crossref] [PubMed]
- Pillman KA, Phillips CA, Roslan S, et al. miR-200/375 control epithelial plasticity-associated alternative splicing by repressing the RNA-binding protein Quaking. EMBO J 2018. [Crossref] [PubMed]
- Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015;160:1125-34. [Crossref] [PubMed]
- Denizli M, Aslan B, Mangala LS, et al. Chitosan Nanoparticles for miRNA Delivery. Methods Mol Biol 2017;1632:219-30. [Crossref] [PubMed]
- Atkinson SJ, Ellison TS, Steri V, et al. Redefining the role(s) of endothelial αvβ3-integrin in angiogenesis. Biochem Soc Trans 2014;42:1590-5. [Crossref] [PubMed]
- Perron G, Jandaghi P, Solanki S, et al. A General Framework for Interrogation of mRNA Stability Programs Identifies RNA-Binding Proteins that Govern Cancer Transcriptomes. Cell Rep 2018;23:1639-50. [Crossref] [PubMed]
- HafezQorani S, Lafzi A, de Bruin RG, et al. Modeling the combined effect of RNA-binding proteins and microRNAs in post-transcriptional regulation. Nucleic Acids Res 2016;44:e83. [Crossref] [PubMed]
- Alkallas R, Fish L, Goodarzi H, et al. Inference of RNA decay rate from transcriptional profiling highlights the regulatory programs of Alzheimer’s disease. Nat Commun 2017;8:909. [Crossref] [PubMed]
Cite this article as: Nehme A, Najafabadi HS, Riazalhosseini Y. A multi-layer post-transcriptional gene regulatory program fuels cancer angiogenesis and metastasis. Biotarget 2019;3:11.


