
Bronchioalveolar stem cells: the crossroads of lung regeneration
Mammalian lungs are encased in dense endothelial cell networks to support systemic delivery of oxygen to vital organs (1-3), and to provide host pathogen defense by physical or chemical removal of foreign bodies (3,4). The evolutionary-refined architecture of mammalian lungs is primarily comprised of endoderm-derived epithelial cell populations (5); however, the lungs also harbour mesoderm-derived cells which contribute to the renin-angiotensin axis (6), innate immunity (3,4), and platelet production (7). Oronasal to parenchymal, the respiratory system is organized from the trachea, bronchi, bronchioles to the alveoli; the primary site of gas exchange (1,2). Gas-exchanging type 1 alveolar (AT1) cells make up most of the alveoli, although surfactant producing type 2 alveolar (AT2) cells can also be distinguished by histology (8). The epithelial lining of the lungs is directly exposed to the environment, and thus must have mechanisms in place to prevent particulate- or pathogen-induced damage (4). The main bronchi are lined with goblet cells that secrete mucus, club cells that detoxify potentially harmful chemicals, and ciliated cells that physically remove foreign particles in a retrograde fashion (Figure 1). Smaller intralobar bronchiole networks demonstrate a similar composition of ciliated and club cells, in addition to neuroendocrine cells that sense environmental changes such as hypoxia (9,10). Considering there is a notable early death associated with respiratory pathologies, such as fibrosis-restricted lung function (11) or underdeveloped lungs in pre-term infants (12), identification of targetable stem cell populations within the lung is critical for developing therapies aimed at pulmonary tissue regeneration (5,13).
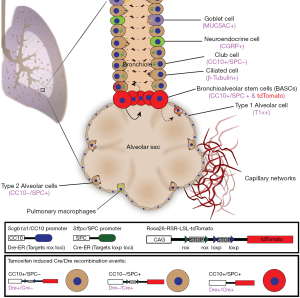
Tissue-specific post-natal stem cells possess 2 defining criteria: the ability to self-renew (to divide without differentiation) and multipotent differentiation into several mature cell types. Several cell populations within the lungs have been reported to exhibit stem cell-like characteristics and contribute to lung homeostasis and regeneration following injury (5,13-15). However, these cell populations were localized to specific regions within the lungs and function specifically to replace epithelial cells; akin to unipotent progenitor cells of the hematopoietic system. For example, naphthalene-resistant club cells represent a source of progenitor cells that regenerate ciliated and non-ciliated epithelial cells in the trachea and bronchioles (5). In contrast, a subpopulation of AT2 cells are restricted to regenerating only alveolar epithelial tissue following bleomycin-induced chemical damage (5). Several populations of cells, such as p63+/cytokeratin5+ cells, have demonstrated the capacity to regenerate both bronchiolar and alveolar cell populations following influenza or bleomycin-induced lung damage (15). However, the homeostatic phenotype of this multipotent cell population has yet to be established using previous lineage-tracing models for fate-mapping analyses. Recently published in Nature Genetics, Liu et al. provided novel evidence of a multipotent stem cell population located at the bronchioalveolar junction (BAJ), that contributes to the homeostasis and regeneration of both bronchiole and alveolar cells in murine lungs following injury (16).
Stem cells within the lung may express multiple mature cell markers at low expression levels (5,14-17), thus limiting the ability of traditional single-lineage transgenic mouse models to resolve targetable stem cells with multipotent function in vivo. Accordingly, Liu et al. generated a dual-lineage reporter mouse using an elegant combination of tamoxifen-inducible Dre-rox and Cre-loxp recombinase systems (Figure 1) integrated within a murine Rosa26-RSR-LSL-tdTomato reporter background (16). Specifically, tamoxifen-inducible Dre recombinase expression was driven under the club cell promoter, Secretoglobin Family 1A Member 1 (Scgb1a1/CC10); whereas, tamoxifen-inducible Cre recombinase expression was driven by the AT2 cell promoter, Surfactant Protein C (Sftpc/SPC). Thus, only cells expressing both CC10 and SPC during tamoxifen induction will express tdTomato through the excision of both stop codons flanked by rox or loxp loci, respectively. This dual-driver transgenic system ensured stringent co-expression within labelled-cells, thus eliminating the labelling of unipotent club and AT2 cell progenitors during lung tissue regeneration. Accordingly, the authors validated the detection of a rare CC10+/SPC+ cells, termed bronchioalveolar stem cells (BASCs), that specifically localized at the BAJ. BASCs were mitotically active yet maintained homeostatic cell frequencies in uninjured mice. Notably, BASCs contributed to the turnover of both club cell and AT2 cells populations during homeostasis, thus suggesting the identification of a functionally-relevant multipotent stem cell. Single-cell RNA-sequencing revealed 2 potentially distinct BASC populations; in addition to markers exclusive to BASC populations compared to committed epithelium, such as Leucine Rich Alpha-2-Glycoprotein 1 (Lrg1) and Perilipin 2 (Pltn2). Lrg1 is commonly expressed in granulocytes and functionally implicated in immunity and angiogenesis (18). In contrast, Perilipin 2 is expressed in a diverse range of cell types and is primarily involved in lipid accumulation (19). Notably, the authors did not observe co-expression of putative lung stem markers p63 and Krt5 in BASCs during homeostasis, possibly reflecting variations in temporal (i.e., tamoxifen half-life) and promoter-specificity of reporter induction and/or variable lung-injury models (naphthalene versus influenza-bronchiole injury) used in each study. The development of similar Dre/Cre transgenic models which investigate the co-expression of CC10 or SPC with additional markers (i.e., Lrg1 or p63) would help to resolve the hierarchical arrangement with previously reported lung stem cell populations. Collectively, BASCs represent a multipotent progenitor cell population with functional roles in lung homeostasis, regeneration, and/or pathology. Therefore, strategies to target this cell population to augment lung tissue regeneration warrants further investigation.
Previous reports have suggested a functional role for CC10+ club cells, identified in CC10 Cre-ER mice (5,16) however, the contribution towards bronchiole cell regeneration by rare CC10+/SPC+ BASCs would be largely underrepresented in the single CC10 transgenic model system. With the new dual-lineage model, Liu and colleagues assessed the functional role of BASCs towards bronchiole cell regeneration following naphthalene exposure injury in 7-week old mice 1-week after tamoxifen induction (16). BASCs maintained homeostatic cell frequencies following injury, however also underwent robust asymmetrical division to generate CC10+/SPC- club cells and acetylated-tubulin+/beta-tubulin+ ciliated cells within the damaged bronchiole epithelium (Figure 2). Notably, the authors did not assess whether BASCs were able to give rise to goblet cells under homeostatic or injury-induced conditions. Nonetheless, BASCs contribution towards AT1, AT2, or CGRP+ neuroendocrine cells following napathalene-induced injury was minimal, assuring napathalene-resistance within BASC and BASC differentiation was a result of direct injury to the bronchiole epithelium. Identification of functional BASCs in vivo provides a foundation to further our knowledge of bronchiole development, pathology, and targeted regeneration.
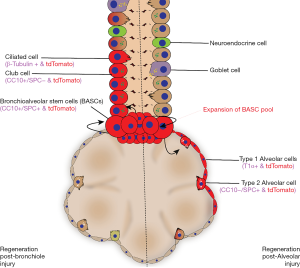
Like the aforementioned CC10-ER transgenic mice studies (5,16), Sftpc-ER transgenic model systems to investigate alveolar tissue regeneration, such as AT2 activation and differentiation, have also been previously performed. Considering, CC10+/SPC+ BASCs were observed to give rise to CC10-/SPC+ AT2 cells and rarely AT1 cells during homeostasis, it warrants further investigation to determine whether multipotent BASCs differentiate towards AT2 “progenitor-like” cells capable of regenerating AT1 epithelium (16). Bleomycin-induced alveolar injury was induced in 7-week old mice to fate-map the contribution of CC10+/SPC+ BASCs to alveolar regeneration. Following alveolar injury, BASCs underwent robust proliferation and generated both AT2 and AT1 cells without giving rise to CC10+ club cells or depleting homeostatic BASC frequencies (Figure 2). Collectively, these observations using different modes of lung injury validate that BASCs demonstrate multipotent cell differentiation uniquely competent to localized injury cues and simultaneously respond through self-renewal to maintain the regenerative cell pool and differentiation to alveolar and bronchiolar cell types. Speculatively, it appears the localization of BASCs to the BAJ provides an exploitable niche to receive activating signals from both proximal and distal epithelial layers with competency to differentiate towards proximal bronchiole tissues or distal alveolar tissues. Notably, RNA-sequencing analysis identified two transcriptionally distinct populations of BASCs, whose functional and individual contributions towards lung regeneration is in need of additional investigations. Liu and colleagues used a confetti reporter fate mapping to determine whether single BASCs were able to regenerate both bronchiole and alveolar tissues (16). However, the authors did not definitively establish whether individual BASCs were intrinsically committed to bronchiole or alveolar regeneration, as simultaneous bronchiole and alveolar lung injury was not assessed. Nonetheless, it remains to be determined whether transcriptionally distinct BASC subsets observed by single cell RNAseq may simply identify dynamic transcription patterns in a homogenous stem cell population with injury-sensitive multipotent contributions during endogenous regeneration.
Creative measures are being taken in regenerative medicine to regenerate endoderm-derived tissues, such as the lung (20) or pancreas (21), that avoid the need for transplantation for cell replacement therapies. For example, bioactive stimuli are secreted from adult progenitor cells may act as a biotherapeutics to regenerate diseased or deleted cell populations and to stimulate angiogenic programs in vivo (20-23). For preterm births at risk of ventilator-induced bronchopulmonary dysplasia, therapeutic strategies that simultaneously promote lung stem cell differentiation and angiogenic microvessel formation would hold significant therapeutic potential to accelerate lung function in vulnerable patients (12,13). Likewise, idiopathic pulmonary fibrosis (IPF) is manifested by a diverse etiology (11,24), including pulmonary hypertension and infections, the pathophysiology of IPF is marked by destruction of both pulmonary and/or alveolar cells, inflammatory macrophage infiltration, fibroblast proliferation and matrix deposition, and regression of tissue-supportive microvascular. Future research will need to determine whether BASCs could be generated ex vivo for subsequent transplantation or whether BASCS are a targetable cell population by cell, gene, or biotherapeutic strategies. Nonetheless, pathologies such as lung cancer have been associated to the BAJ (25), thus multipotent and mitotically-active BASCs provide a potential cell of origin for tumor formation. Recently developed organoid culture systems will allow for high throughput drug screens to identify biological targets for applications of regenerative medicine and anti-cancer therapies. Collectively, more research is required to fully understand the extent to which BASCs contribute to lung homeostasis, regeneration and pathology. The work presented by Liu and colleagues provides foundational insight towards a potentially exploitable lung-resident stem cell residing at the crossroads of lung regeneration.
Acknowledgements
Funding: This work was supported by an operating grant from the Canadian Institute of Health Research (CIHR) (MOP# 378189) and Juvenile Diabetes Research Foundation (2-SRA-2015-60-Q-R).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- deMello DE, Sawyer D, Galvin N, et al. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 1997;16:568-81. [Crossref] [PubMed]
- Clements JA. Functions of the alveolar lining. Am Rev Respir Dis 1977;115:67-71. [PubMed]
- Fishman AP. Nonrespiratory functions of the lungs. Chest 1977;72:84-9. [Crossref] [PubMed]
- LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect 2001;3:161-6. [Crossref] [PubMed]
- Hogan BL, Barkauskas CE, Chapman HA, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014;15:123-38. [Crossref] [PubMed]
- Pieruzzi F, Abassi ZA, Keiser HR. Expression of renin-angiotensin system components in the heart, kidneys, and lungs of rats with experimental heart failure. Circulation 1995;92:3105-12. [Crossref] [PubMed]
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017;544:105-9. [Crossref] [PubMed]
- Stone KC, Mercer RR, Gehr P, et al. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol 1992;6:235-43. [Crossref] [PubMed]
- Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr Respir Rev 2001;2:171-6. [Crossref] [PubMed]
- Hage E, Hage J, Juel G. Endocrine-like cells of the pulmonary epithelium of the human adult lung. Cell Tissue Res 1977;178:39-48. [Crossref] [PubMed]
- Kärkkäinen M, Nurmi H, Kettunen H-P, et al. Underlying and immediate causes of death in patients with idiopathic pulmonary fibrosis. BMC Pulm Med 2018;18:69. [Crossref] [PubMed]
- Thébaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology 2007;91:291-7. [Crossref] [PubMed]
- Kang M, Thébaud B. Stem cell biology and regenerative medicine for neonatal lung diseases. Pediatr Res 2018;83:291-7. [Crossref] [PubMed]
- Kumar PA, Hu Y, Yamamoto Y, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011;147:525-38. [Crossref] [PubMed]
- Zuo W, Zhang T, Wu DZA, et al. p63+ Krt5+ distal airway stem cells are essential for lung regeneration. Nature 2015;517:616-20. [Crossref] [PubMed]
- Liu Q, Liu K, Cui G, et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 2019;51:728-38. [Crossref] [PubMed]
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173-82. [Crossref] [PubMed]
- Wang X, Abraham S, McKenzie JA, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013;499:306-11. [Crossref] [PubMed]
- Greenberg AS, Egan JJ, Wek SA, et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 1991;266:11341-6. [PubMed]
- Lesage F, Joseph J, Alphonse RA, et al. Endothelial colony-forming cell-derived exosomes attenuate pulmonary hypertension and hypoplasia in neonatal rats. J Extracell Vesicles 2018;7:208.
- Kuljanin M, Elgamal RM, Bell GI, et al. Human multipotent stromal cell secreted effectors accelerate islet regeneration. Stem Cells 2019;37:516-28. [Crossref] [PubMed]
- Ishizawa K, Kubo H, Yamada M, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249-52. [Crossref] [PubMed]
- Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 2014;20:822-32. [Crossref] [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Kim CFB, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35. [Crossref] [PubMed]
Cite this article as: Cooper TT, Hess DA. Bronchioalveolar stem cells: the crossroads of lung regeneration. Biotarget 2019;3:8.


