
From prevention to cure, repurposing anti-viral vaccines for cancer immunotherapy
Human papillomaviruses (HPVs), of which there are more than 200 types, infect epithelial cells of the skin and mucosal surfaces by direct contact and, as a whole, are fairly ubiquitous in the human population. These viruses are intraepithelial pathogens that enter but do not kill the host cell and have evolved numerous mechanisms to evade the host immune system. Of clinical significance, over five percent of human cancers are initiated by infection with HPV. These include cervical, oropharyngeal, anal, penile, vulvar, and vaginal cancers, which are driven by sexually transmitted HPVs, types 16 and 18. The incidence of cervical cancer, the most common women’s cancer worldwide, has decreased in the western world following widespread implementation of expensive “Pap” screening programs paired with early clinical intervention. Certain HPV-driven cancer types are however on the increase, including oropharyngeal and anal cancers, especially in men. Additionally, a significant fraction of cutaneous squamous cell carcinomas (cSCC) and basal cell carcinomas (cBCCs) are associated with HPV 5 infection or with the dozen or so other cancer-causing HPV types (1,2).
The pursuit of an anti-HPV vaccine was driven by the story of cervical cancer, wherein >70% of tumors are caused by HPV16/18 infection, and over 90% by some type of oncogenic HPV. With the development of the anti-HPV prophylactic vaccine, Gardasil® (3), and the introduction of large scale childhood vaccination programs, HPV-driven cancers could virtually be eliminated within the next 20–30 years. Nevertheless, it is estimated that there will still be millions of new cases of cervical and other HPV-driven cancers arising over the next few decades in patients already infected with HPV, or who have not received the vaccine. The need for therapeutics that treat HPV-induced cancers will, therefore, persist for years to come.
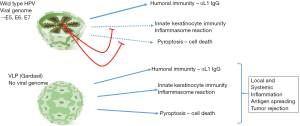
Mature HPV virions consist of non-enveloped icosahedral capsids that encapsulate DNA encoding the viral genes. The capsid is composed of 360 molecules of L1 capsid protein organized into 72 pentamers and associated with a single L2 capsid protein per pentamer (Figure 1). During vaccine development, it became apparent that the HPV viral capsid proteins showed enhanced immunogenicity when delivered as a virus-like particle (VLP), which is essentially a self-assembly of viral capsid proteins lacking viral genetic material (Figure 1) (1,3). First generation Gardasil® was such a VLP preparation, composed of the assembled HPV capsid proteins, L1 and L2. This quadrivalent vaccine was composed of L1 proteins from HPV types 6, 11,16 and 18, and was FDA-approved as a prophylactic agent in 2006. In 2014, the most advanced HPV vaccine, Gardasil®9, that immunizes against nine different HPV types, was also FDA-approved and is now the only such vaccine in use in the USA. Gardasil®9 protects against the most oncogenic HPVs types HPV 16 and 18, as well as HPV 6 and 11 that cause genital warts, plus five other cancer-causing HPVs (31, 33, 45, 52, and 58). The vaccine is a complex of L1 proteins from these nine viral types that self-organize into highly immunogenic VLPs when expressed from yeast or insect cell lines. Gardasil®9 immunization results in a humoral immune response to the L1 capsid protein, and circulating anti-L1 IgG. The vaccine is most effective when administered before initial contact with HPV, hence the target population is 11–12-year-old children. Nevertheless, the vaccine does confer some protection in older people, even in those previously exposed to HPV, and FDA approval of vaccination for individuals up to 45 years of age was announced in October 2018 (FDA.gov press release).
A Brief Report in JAMA Dermatology recently suggested that Gardasil®9 might also have benefit for cancer treatment (4). This report (4) documented a clinical case study suggesting potential cancer therapeutic activity of Gardasil®9 against established cutaneous basaloid squamous cell carcinomas (BSCCs). BSCC, in general, is an uncommon tumor type, and cutaneous BSCC is even rarer, but known for its highly invasive growth, recurrence after surgical resection and known predilection towards metastasis (5). It was somewhat remarkable, therefore, that the patient in this study who, in her 10th decade, presented with multiple unresectable cutaneous BSCCs, responded well to Gardasil®9 therapy, attaining complete and durable regression of all carcinomas following treatment (4). The therapy consisted only of two intramuscular (systemic) doses of Gardasil®9 given 6 weeks apart, followed 3 weeks later by intra-tumoral vaccine injections into the three largest tumors, and two repeat intratumoral administrations over the next 8 months. By the end of treatment, all tumors, not only those that had been injected, showed complete and durable regression with no local or distant tumor recurrence in the 18-month period of follow up, thus implicating a systemic and/or immune-mediated effect. That report presents the first clinical evidence that this normally prophylactic anti-HPV vaccine, may be of use as an immunotherapeutic agent for cancer (4).
Many questions are raised by this anecdotal study of one patient, not least whether this was a fortuitous case of spontaneous immune-mediated tumor regression, as has been seen for low and high risk squamous intraepithelial lesions of the cervix (1) and isolated cases of malignant melanoma and other tumor types (6). Nevertheless, the results show sufficient promise to extend this treatment to more patients bearing unresectable SCCs, in order to estimate anti-tumor efficacy. Importantly, Gardasil®9 has been used to vaccinate more than 30 million people in the USA alone and has shown excellent safety when administered intramuscularly for induction of a humoral immune response against the HPV viral capsid protein. Following vaccination, there is unequivocal evidence of reduced HPV infections and early cervical dysplasias that associate with later onset of cervical cancer (3).
If found to be of general therapeutic utility, intratumoral Gardasil®9 inoculations could have major implications for the use of this vaccine to treat established carcinomas, particularly unresectable cSCC, and SCC of the head and neck (SCC-HN). In the US, cSCC is now the most common human cancer exceeding 250,000 new cases per year (7). However, this disease has frequently been misconceived as having a low clinical impact because, in the majority of patients, cSCCs can be controlled by surveillance and early intervention. Nevertheless, although 98% of cSCC are manageable by surveillance, around 2% of cSCCs in the general population will metastasize, ultimately leading to death (8). In transplant recipients on anti-rejection drugs, an increasing demographic due to the successes of organ and stem cell transplant therapies, rates of cSCC are 65-fold higher than in the general population (9). This is in part due to reduced tumor immuno-surveillance, but also due to a higher rate of HPV infection. cSCCs that arise in transplant recipients tend to be more aggressive, with a metastasis rate of 6–7% in adults and ~13% for those that occur in pediatric transplant recipients (9). On an annual basis, therefore there are as many deaths from cSCC as there are from cervical cancer or melanoma. Notably, melanoma is far less prevalent than cSCC, but has a higher fatality rate. cSCC is therefore a disease whose treatment could be transformed by Gardasil®9 immunotherapy.
With the assumption that Gardasil®9 can be validated as a therapeutic modality in additional cancer patients, questions that arise include whether successful outcomes depend on tumor HPV status, whether some epithelial tumor types may be more responsive than others, and what are the predictors of response? Most importantly, for reasons of both safety and efficacy, the mechanism of action of Gardasil®9 as a therapeutic modality should be elucidated. Gardasil®9 was designed to generate a productive anti-L1 humoral immune response that prevents viral entry into the epithelial cell (2,3). How then does this same vaccine elicit an anti-tumor response when inoculated intratumorally? Clearly, we cannot begin to answer these questions on the basis of a single patient, but we can propose possible mechanisms based on current knowledge of anti-HPV immunity and information learned from other anti-viral vaccine programs that have been re-purposed as cancer therapeutics.
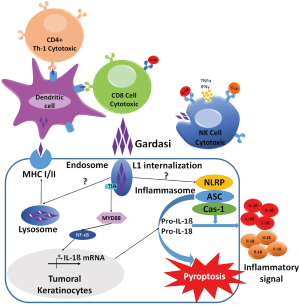
Both wild-type HPVs and Gardasil®9 VLPs induce anti-L1 humoral immune responses, albeit that the response to systemic Gardasil®9 vaccination is over forty times stronger than that to innate epithelial HPV infection (1,2). Both HPV and VLPs can be taken up by NK cells, to elicit an inflammatory response (10), and in HPV-positive BSCCs, circulating anti-L1 IgGs may opsonize inoculated VLPs, causing FCγR-mediated activation of NK cells and uptake by phagocytic macrophages, thereby boosting inflammation within the vicinity of the tumor. Additionally, both HPV and VLPs can enter regenerative keratinocytes (11,12). No defined receptor for entry of the HPV L1 capsid protein has yet been identified and may not even be required. Viral or VLP uptake requires binding to basement membrane components, occurs through macropinocytosis into epithelial cells of the basal layer, and uptake appears to be dependent on a regenerative state, for examples in response to abrasion (1,2). Following viral uptake, and lysosomal degradation, L1 neoantigens can be presented by MHCI and MHCII antigen presentation complexes on the surface of infected epithelial cells. This leads to cross-presentation to professional antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs), to potentiate an adaptive and systemic immune response (Figure 2). In the paper in question (4), HPV status of the BSCC patient was not reported, although very high p16Ink4A expression levels hint at a virally driven mechanism (1). Based on knowledge gained from studies on other anti-viral vaccines developed as cancer therapeutics, as well as by studies to develop oncolytic viruses, it is also possible that prior HPV exposure and L1 sera-positivity may not be required for Gardasil®9-initiated carcinoma eradication.
The idea of using microbial products as therapeutic agents was conceived by Coley in the late 19th century, who found that repeatedly injecting live Streptococcal bacteria into tumors could lead to tumor regression (13). This concept was further developed in the early to mid-20th century by Pearl (14) and Old (15), specifically approaching the issue from the perspective of cancer immunotherapy. During human autopsies, Pearl made the critical observation that individuals with tuberculosis infection had a significantly reduced incidence of cancer (14). Old followed this observation up by showing experimentally that vaccination with Bacillus Calmette-Guerin (BCG, the causative bacteria for tuberculosis) protected mice from ensuing transplantation with tumor cells, and that this required live bacteria and an active immune system (15). The BCG vaccine has now been used for over 30 years as an anti-cancer therapeutic for bladder cancer, with a significant survival benefit for patients, albeit with occasional undesirable side effects (16).
Epithelial uptake of BCG, like that of HPV, occurs by macropinocytosis (16), we can therefore apply concepts learned from the BCG field to the study of HPV. Notably, BCG researchers showed that macropinocytosis, the innate process of nanoparticle uptake by cells, is positively regulated by cancer-causing mutations such as loss of PTEN or activation of KRAS (16). Since Gardasil®9 VLP, just like its parental HPV virion, is also taken up by macropinocytosis (11,12), the L1 capsid should preferentially be taken up by malignant rather than normal cells. Once inside the tumor cell, L1 capsid protein is delivered to, and degraded within the endo-lysosomal system, such that highly immunogenic L1 peptides have the potential (I) to be presented on the tumor cell surface as MHCI and/or MHCII L1 neo-antigens, and (II) to be detected as pathogen-associated molecular patterns (PAMPs) by pathogen recognition receptors (PRRs) within the keratinocyte.
Inside the cell, ligation of L1 PAMPs with their PRRs, such as Toll-like receptors (TLRs), nucleotide binding oligomerization domain-like receptors (NLRs), and retinoic acid-inducible gene I-like receptors (RLRs), would initiate distinct intraepithelial cell inflammatory programs. TLRs ligated within the endosomal compartment cause activation of Mal/Myd88- or TRIM/TRIF-mediated host signaling pathways, to initiate both innate and adaptive immune responses, namely production of type I interferons and cytokines that initiate CD4+ Th1-type cytotoxic responses (Figure 2). In parallel, activation of NLRs results in expression of pro-caspase 1. NLRP3 and NLRP1 also nucleate the assembly of inflammasomes which are active in human keratinocytes (17,18). Inflammasomes then instigate cleavage of procaspase 1 to active caspase 1 that in turn cleaves pro-interleukin 1β (pro-IL-1β) and pro-IL-18 into their bioactive forms (19). Keratinocytes are therefore induced to secrete several cytokines, including IL-1, IL-6, IL-10, IL-18, and tumor necrosis factor α, with IL-1β being central to the activation of CD4+ Th cells and DCs. Significantly, the inflammasome also activates pyroptosis, a form of cell death that ruptures the cell to release more tumor antigens that amplify the inflammatory processes.
Importantly, during infection with wild-type HPV, these inflammatory processes and cell death are potently inhibited by virally-encoded oncogenes that execute highly immuno-suppressive and anti-apoptotic programs (Figure 1). Viral oncogenes act at multiple signaling nodes within the keratinocyte to evade both the innate and adaptive host immune systems. For example, the HPV E7 viral oncogene inhibits IFN-α-mediated signal transduction by binding and cytoplasmic retention of an IFN-α signaling component, P48/IRF-9, thereby preventing the formation of the transcriptional complex that activates interferon-specific response elements (20). E7 also associates with IRF-1 to inhibit IRF-1-mediated activation of the IFN-β gene promoter. Inhibition of IRF-1 also suppresses the expression of genes encoding components of the antigen presentation machinery, such as TAP1, and macrophage chemoattractant protein 1 (MCP-1) (20). HPV infection also causes immunosuppression by increasing MHCII expression and supporting immunosuppressive Treg expansion (21,22). Neutralization of Treg activity or transdifferentiation of CD4+ Tregs towards Th cells, such as by treatment with α-TGFβ (23) may therefore be considered an additional or complementing approach to treat HPV-positive SCC regression.
A thought experiment, however, suggests that HPV sera-negative cancer patients may respond to Gardasil as well, if not better, than those with HPV positive tumors, since the latter would be immunized against VLP uptake by tumor cells. Unimpeded by anti-VLP IgG, tumor cell uptake of VLP would allow L1 and its degradation products to be recognized as PAMPs, initiate the PRR-mediated inflammatory pathways, inflammasome assembly, and pyroptosis, all without hindrance from the immunosuppressive activity of E7 and E6 oncoproteins (Figure 1). This hypothetical model of activation of the immune system by VLP would provide three key elements of a successful oncolytic virus (24), namely specificity for uptake by tumor rather than normal epithelial cells, tumor cell death necessary for release of tumor antigens, and engagement of the host cellular innate and adaptive immune systems through antigen presentation. Although not designed as such, Garadsil®9 might therefore turn out to be an effective tumoricidal agent, that acts preferentially against tumor versus normal keratinocytes to initiate cellular inflammation and induce malignant keratinocyte cell death. Tumor cell death need only occur in a few tumor cells, as the release of additional tumor antigens that could potentiate tumor immune rejection by uptake and cross-presentation to professional APCs, macrophages and DCs, and consequent activation of CD8+ cytotoxic T cells and CD4+ cytotoxic Th cells. Subsequent cycles of tumor cell death and immunity would thereby amplify the diversity of tumor antigens against which T cells react, leading to the phenomenon of “neoantigen spreading” and efficacious tumor rejection (25). Thereby, cytotoxic CD8+ T and CD4+Th cells that ultimately eliminate the tumor may not be specific for the initiating HPV L1 protein, but for some other tumor neoantigen. In fact, neoantigen spreading has been shown to contribute to the elimination of genetically heterogeneous tumors, such as SCCs (25).
With widespread implementation of the Gardasil®9 prophylactic vaccination program, cervical and other HPV-driven cancers have the potential to be eliminated. However, the demand for efficacious HPV-associated cancer therapeutics will not immediately disappear, since such cancers take decades to develop and the vast majority of individuals to date have not received this vaccine. Moreover, patient resistance to vaccination programs can be a major hurdle to any such public health program.
Acknowledgments
Funding: The Akhurst Laboratory is supported by NIH/NCI grants: 5P30CA082103, R01CA210561, and R01HL122869.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Executive Editor-in-Chief Dr. Hualin Sun (Jiangsu Key Laboratory of Neuroregeneration, Nantong University, Nantong, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/biotarget.2018.12.03). RJ Akhurst has sponsored research agreements with Pfizer Corp. and Plexxikon. RJ Akhurst is a co-inventor of US patent (No. 10.167,334) and International Patent #PCT/US2016/025802 co-owned by UCSF and Xoma; Mirza et al. Treatment of Cancer Using Inhibitors of TGF-beta and PD-1—2015 and WIPO Patent Application WO/2016/161410A2 U.S. Prov. Utility Appl. No. 62/143,016; A Mirza, O Li, R Akhurst. O Mamaï is a co-inventor of patent application WO 2018/056907 A1, Novel Target and Methodology to Inhibit Skin Inflammation and Blunt Skin Cancer Susceptibility. Owned by A*STAR (Singapore). The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342-50. [Crossref] [PubMed]
- Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev 2012;25:215-22. [Crossref] [PubMed]
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711-23. [Crossref] [PubMed]
- Nichols AJ, Gonzalez A, Clark ES, et al. Combined Systemic and Intratumoral Administration of Human Papillomavirus Vaccine to Treat Multiple Cutaneous Basaloid Squamous Cell Carcinomas. JAMA Dermatol 2018;154:927-30. [Crossref] [PubMed]
- Linton OR, Moore MG, Brigance JS, et al. Prognostic significance of basaloid squamous cell carcinoma in head and neck cancer. JAMA Otolaryngol Head Neck Surg 2013;139:1306-11. [Crossref] [PubMed]
- Everson TC. Spontaneous regression of cancer. Ann N Y Acad Sci 1964;114:721-35. [Crossref]
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 2005;26:1657-67. [Crossref] [PubMed]
- Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 2013;149:541-7. [Crossref] [PubMed]
- Ajithkumar TV, Parkinson CA, Butler A, et al. Management of solid tumours in organ-transplant recipients. Lancet Oncol 2007;8:921-32. [Crossref] [PubMed]
- Da Silva DM, Fausch SC, Verbeek JS, et al. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcgamma receptors and contributes to acquisition of T cell immunity. J Immunol 2007;178:7587-97. [Crossref] [PubMed]
- Volpers C, Unckell F, Schirmacher P, et al. Binding and internalization of human papillomavirus type 33 virus-like particles by eukaryotic cells. J Virol 1995;69:3258-64. [PubMed]
- Muller M, Gissmann L, Cristiano RJ, et al. Papillomavirus capsid binding and uptake by cells from different tissues and species. J Virol 1995;69:948-54. [PubMed]
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci 1893;105:487-510. [Crossref]
- Pearl R. Cancer and tuberculosis. Am J Epidemiol 1929;9:97-159. [Crossref]
- Old LJ, Clarke DA, Benacerraf B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature 1959;184:291-2. [Crossref] [PubMed]
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 2014;11:153-62. [Crossref] [PubMed]
- Sand J, Haertel E, Biedermann T, et al. Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes. Cell Death Dis 2018;9:24. [Crossref] [PubMed]
- Zhong FL, Mamai O, Sborgi L, et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016;167:187-202.e17. [Crossref] [PubMed]
- Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci 2017;130:3955-63. [Crossref] [PubMed]
- Um SJ, Rhyu JW, Kim EJ, et al. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett 2002;179:205-12. [Crossref] [PubMed]
- Ao C, Zeng K. The role of regulatory T cells in pathogenesis and therapy of human papillomavirus-related diseases, especially in cancer. Infect Genet Evol 2018;65:406-13. [Crossref] [PubMed]
- Chuang CM, Hoory T, Monie A, et al. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine 2009;27:684-9. [Crossref] [PubMed]
- Dodagatta-Marri E, Meyer DS, Reeves MQ, et al. Elevated pSmad3 signaling and Treg levels induced by a-PD1 therapy are targeted by TGFβ blockade to promote regression and durable immunity of high mutation carcinomas. J Immunother Cancer 2018; In Press.
- Hamid O, Hoffner B, Gasal E, et al. Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother 2017;66:1249-64. [Crossref] [PubMed]
- Gulley JL, Madan RA, Pachynski R, et al. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
Cite this article as: Mamaï O, Dodagatta-Marri E, Akhurst RJ. From prevention to cure, repurposing anti-viral vaccines for cancer immunotherapy. Biotarget 2018;2:20.


