
CAR-T cells shed light on the treatments of fatal liver diseases
Introduction
In the field of liver diseases, viral hepatitis and liver carcinoma (hepatic carcinoma) are interrelated diseases, because their main leading cause is due to hepatitis virus infection (1,2). The numbers of patients with viral hepatitis and liver carcinoma are enormous, special in China. Hepatic carcinoma has a relatively high mortality rate, and the death tolls increased year by year. In 2006, in Lancet Stanaway pointed out that viral hepatitis had become one of the world’s leading causes of death and disability, and the death toll per year was at least equal to tuberculosis (TB), malaria and acquired immune deficiency syndrome (AIDS) (3). During the 23 years from 1990 to 2013, up to 96% of deaths from viral hepatitis were caused by hepatitis B virus (HBV) and C virus (HCV), accompanied with secondary liver cirrhosis and hepatocellular carcinoma (HCC) (3). The occurrence and progression of liver cancer is closely related to HBV or HCV infection, accounting for about 80% in the etiology of liver cancer (4). Currently, hepatectomy is the most direct treatment for HCC. However, most patients with HCC are usually diagnosed at an advanced stage, and conventional therapies (including surgery, local ablation, interventional therapy, radiotherapy, and chemotherapy) have a high recurrence rate and a poor prognosis (5). Sorafenib, the first new target drug approved by US food & drug administration (FDA) for the treatment of unresectable HCC, can prolong overall survival by 2 to 3 months (6). Consequently, whether for viral hepatitis or liver cancer, it is urgent to search for a new therapeutic strategy.
T lymphocytes, as the core immune cells that mediate adaptive cellular immune responses, could kill tumor cells or host cells infected by viruses and other pathogens. In recent years, researchers have genetically modified T cells to express chimeric antigen receptor (CAR), known as CAR-T cells, evolving the ordinary t cells to recognize specific antigen (7,8). On the basis of the original function, T cells can bypass antigen presenting cells to kill target cells in non MHC restricted manner, and enhance the specificity of T cells to accurately kill abnormal cells (8). In addition, CAR-T cells were also personalized drugs that have the function of self-amplification and memory. So, CAR-T could be used in the continuous drug treatment for long time (9,10). It can be said that CAR-T cells open up a new era of immune therapy and provide new opportunities for the treatment of viral hepatitis and liver cancer. In this review, we focused on the preclinical and clinical progress in the CAR-T therapy of liver disease, and compare the difference of CAR-T application between hematologic malignancies and liver cancer. It will give a new insight for researchers to seek new approache to improve clinical outcomes of CAR-T cell therapy in liver diseases in the future.
CAR structure and characterization
T cells are genetically modified to express a CAR that can specifically recognize tumor specific antigen. Furthermore, CAR is a fusion structure formed by a simulation of physiological function of T cell receptor (TCR). The CAR is mainly composed of four parts, extracellular antigen recognition domain [usually derived from single chain variable fragment (scFv) of monoclonal antibody (mAb)], extracellular spacer domain [such as the hinge region of human immunoglobulin G (IgG)] (11), transmembrane domain (connection and fixation) and intracellular signal transduction domains. The structure diagram of CAR was showed in Figure 1. The extracellular spacer domain was often ignored due to the lack of signaling function. However, some studies had shown that adjusting its length and structure could optimize its specificity, T cell proliferation and promoting cytokine production (11), in result some attention had been drawn recently.
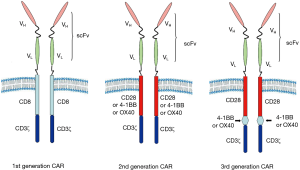
Benefit from the continuous advance in the modification of the intracellular domain, multiple generations of CAR have been developed. The first generation CAR contains only one CD3ζ cytoplasmic region of TCR/CD3 complex. Without sufficient second signal, targeting tumor T cells were easy to initiate anergy, reduce cytokine secretion and even apoptosis because of lack of T cell co-stimulation (12). In order to overcome the defects of the first generation CAR, the second generation CAR was fused one co-stimulatory signal domain (such as CD28, OX40 or 4-1BB) (Figure 1), allowing the simultaneous transmission of the first and second signals (13). By comparing the clinical treatment outcomes of the first and second generation CAR, it was confirmed that the second generation CAR had longer persistence in vivo (14). Meanwhile, a large number of clinical trials have been carried out utilizing the second generation CAR fused with CD28 or 4-1BB signaling domains. These results demonstrated that CAR fused with CD28 could be amplified more quickly, whereas CAR fused with 4-1BB had a longer persistence (15). Among them, the third generation of CAR, containing CD28-4-1BB or CD28-OX40 co-stimulatory domain, has been shown to permanently activate T cells, but the efficiency remains to be assessed (16,17). Recently, more and more researchers began to pay attention to the fourth generation CAR (also known as TRUCK) that additional integrates transgenic immune factors (mainly IL12) or co-stimulatory factor ligands (4-1BBL, CD40L) to induce long-lasting anti-tumor response (18). To date, the second generation CAR is still commonly used in clinical trials.
It was convinced that the CAR-T cell therapy was particularly useful in the treatment of B cell malignancies and had achieved encouraging clinical efficacy and breakthroughs. Among them, CD19 CAR-T cells exhibited an amazing efficacy in hematologic malignancies (10,19-21). In fact, the initial constructed CAR-T cell (with the ability of targets identification and signal transduction) was not for the treatment of cancer (22). The initial CAR-T cells could specifically recognize and lyse human immunodeficiency virus (HIV)-infected CD4+ T cells (22). The interleukin 13 receptor alpha 2 (IL13Rα2) is a membrane bound protein and glioma-restricted cell-surface epitope. The first application of CAR-T cell for solid tumor was IL13Rα2 CAR constructed for glioma by Jensen (23). In human glioblastoma orthotopic xenograft of mouse, IL13Rα2 CAR-T cells showed the regression of xenograft and the potential for the treatment of solid tumors (23). Yet, the results of several clinical studies are not ideal. In recent years, researchers have accelerated progress in the field of utilizing of CAR-T for solid tumor research, promoting this promising therapy for ovarian cancer (OC) (24), renal cell carcinoma (25), colorectal cancer (26), HCC (27), bile duct cancer (28), gastric cancer (29), and other refractory cancer. Furthermore, a few studies have also used this effective method for treatment of HBV (30,31) and HCV (32). So, we believe without doubt that CAR-T cell therapy will open up more areas of application of CAR-T and give researchers more inspiration in the future.
Appropriate targets and preclinical studies
Compared with application of CAR-T in hematological malignancies, the utilization of CAR-T cell therapies for liver diseases is still few. Suspended at the top of the cause list is the absence of a suitable target. In CAR-T therapy, TAA is the key to specific recognition for tumor cells. It is a dilemma that most TAAs are not only expressed on the surface of tumor cells, but also on the surface of normal cells with different expression level. Cancer is a disease caused by gene mutations in normal cells, so it is easy to explain. Other more, many TAAs exist in intracellular and are not appropriate for conventional CAR-T cells that can only recognize cell surface antigens. As a result, the number of available TAA is limited and it is difficult to find an ideal target. Herein, the appropriate targets of previous application and ongoing preclinical studies in CAR-T therapy were summarized and the possible reasons for these TAAs selection for viral hepatitis, HCC and metastatic liver cancer were analyzed.
Viral hepatitis
The hepatitis B surface antigen (HBsAg) is a HBV surface protein and produced persistently in infected cells via HBV replication (33). In addition, after the long-term highly effective antiviral treatment, a large number of hepatocytes (5–30%) remained HBsAg positive, even in advanced stages of chronic hepatitis B (HCC has developed) (34). Therefore, it seems appropriate to target HBsAg. It was reported by Bohne in 2008 that scFv-based HBsAg specific CAR-T cells could recognize HBV and kill HBV associated HCC (31). The report suggested that a promising novel therapeutic approach for the treatment of HBV. Further evidence was that HBsAg CAR-T could inhibit HBV replication, but cause only transient liver damage via adoptive transferring to CD45.2+ HBV transgenic mice (30). Therefore, further preclinical trials are needed to assess the safety of this treatment.
HBV E1 and E2 proteins are viral envelope glycoprotein, and HCV E2 proteins are more immunogenic. In addition, current evidence that anti HCV E2 mAb could inhibit intercellular transmission of HCV indicated that HCV E2 could serve as a good target (35,36). Over the past few years, many anti HCV E2 mAb have been evaluated as potential candidates for antiviral drugs in preclinical and clinical trials (37). Sautto screened out high affinity mAb targeting HCV E2 conserved region to construct CAR-T cells, which were capable of releasing interferon gamma (IFN-γ), interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α) and ultimately lysing HCV infected hepatocytes (32). It can be concluded that targeting HCV E2 glycoprotein CAR-T therapy is a promising route for clearing HCV infected cells, opening future prospects for the treatment of currently unresponsive patients with antiviral drugs.
HCC
Glypican 3 (GPC3), as a member of the Glypican gene family, is a membrane heparan sulfate proteoglycan, closely associated with cell growth, differentiation and tumor progression (38). Based on the experimental knockdown results, knockdown of GPC3 could induce apoptosis of hepatoma cells, indicating that GPC3 was a crucial gene of HCC cells (39). What’s more, GPC3 was highly expressed in 75% of HCC samples and was lowly expressed in healthy livers or other normal tissues (40,41). Therefore, as a valid target, compared to other targets, GPC3 has greater security.
Previously, it was confirmed in the cell line-derived xenograft (CDX) model and the patient-derived xenograft (PDX) model that the third generation CAR-T cells targeting GPC3 could effectively inhibit the growth of liver cancer and reduce the tumor volume (42,43). Two years later, on the basis of previous studies, Li constructed a dual-targeted CAR-T cell capable of targeting both GPC3 and asialoglycoprotein receptor 1 (asgr1). The results showed that the cytotoxic and targeting ability of dual-targeted CAR-T cell was better efficacy than that of conventional CAR-T cells (44). Furthermore, at the American association for cancer research (AACR) annual meeting in 2016, Trinh first proposed that Sorafenib and the fourth generation GPC3 CAR-T cell combined therapy could increase the specific lysis by 25% (45). The novel combined therapy provided data support for the new strategy of clinical treatment of HCC, but the safety was still need to be assessed. Further studies on the CAR-T cell therapy for HCC require constant innovation and improvements.
Mucin 1 (MUC1), which is a glycoprotein, plays an important role in the tumorigenesis and progression of many adenocarcinomas (46). Under normal physiological, MUC1 was mainly expressed in the epithelial cells near the lumen or gland cavities of various tissues and organs, with polarity distribution. MUC1 is aberrantly upregulated expressed in various tumors, and widely distributed. Recent studies had indicated that MUC1 could promote the proliferation and migration of HCC cells (47,48). It was demonstrated the feasibility of MUC1 as a target, and also the superiority of the third generation CAR-T cells by comparing the cytotoxic ability of the first and third generation of MUC1 CAR-engineered Jurkat T cells (49). But in this study, the gene-modified Jurkat T cells was used, a kind of tumor cell lines, not entirely representative of human T cells, which has limited the impact of the research to some extent.
Alpha fetoprotein (AFP) is a secreted glycoprotein and usually overexpressed in tumors (including HCC) originating from the endoderm (50,51). Clinically, AFP is used as a cancer marker for screening HCC and assessing the liver function in patients with HCC (52). However, AFP is an intracellular/secreted protein that is not expressed on the surface of HCC cells and can’t be targeted by conventional CAR-T cells. Creatively, unlike traditional CAR-T cells, novel CAR-T cells were designed to target the AFP peptide-MHC complex, making it possible to target the intracellular antigens and vastly expanding the range of targets (53).
CD133 is a five transmembrane glycoprotein used as a biomarker for identifying cancer stem cells (54). In addition, studies had shown that a few CD133 positive hepatocarcinoma stem cells existed in human HCC, resulting in high tumorigenicity, low survival rates and poor prognosis (55,56). CD133 CAR-T and CD133 CAR-engineered NK92 cells could specifically kill patient-derived glioblastoma stem cells and CD133-positive OC cells respectively (57,58). Although there is not still a CD133 CAR-T cells for HCC so far. We have a conviction that a targeting CD133 CAR for HCC is coming.
Liver metastases (LM)
Carcinoembryonic antigen (CEA), which is a set of highly related glycoprotein, is highly expressed on the cell surface of many human epithelial tumors, originally found in colon cancer (59). Although CEA is expressed in some normal epithelial cells, CEA always exists on the apical membrane of the epithelial cells (60). In contrast, tumor cells will lose the apical polarity of CEA expression (61). The CEA may enter the capillaries and circulate with the blood, leading to elevated serum levels of soluble CEA. Based on the founding, CEA can be used as an excellent biomarker for cancer identification and an appropriate TAA target for cancer immunotherapy (62). LM develop in more than 50% of colorectal cancer patients, with a certain expression of CEA (63). Fortunately, we can treat LM by targeting CEA. In 2008, the second generation CAR-T cells with CD28 as a co-stimulatory domain were constructed for the first time, targeting CEA positive tumor cells and showing an effective anti-tumor effect in the patients with LM (64). But unfortunately, the immunosuppressive cells [such as myeloid-derived suppressor cells (MDSC)] in the liver cause tumor cells to evade immune surveillance, without effective immune response (65). In 2015, using anti-CEA CAR-T cells in the CEA+ LM mouse model, the mechanism about hepatic MDSC amplification and inhibition of CAR-T cells function was identified (66). Thus, in the future, highly specific CAR-T cell therapy combined with inhibition of immunosuppressor cell from the intrahepatic tumor microenvironment can be one possible approach to the treatment of LM.
Past research has shown that MG-7 Antigen (Ag) is a kind of gastric cancer associated Ag with high specificity, highest rate of Ag expression in gastric cancer (67). At the AACR annual meeting in 2016, Jijun Yuan reported that MG-7 Ag is the very specific Ag of gastric carcinoma and can be utilized as a new therapeutic target for CAR-T cell therapy. The liver is the most common metastatic site of gastric cancer, which will lead to LM probably (68). Appropriate therapies for liver metastasis are rare, only surgical excision, transarterial chemoembolization and systemic chemotherapy (69-71). The emergence of CAR-T cell therapy offers new hope for liver metastasis in gastric cancer.
Recent progress in clinical practice
It is conceivable that the premise for CAR-T cells to exert the greatest effect in clinical is effective preparation. The preparation of CAR-T cells can be roughly divided into five steps: (I) the collection of autologous T cells (including a variety of T cell subsets); (II) gene modification by viral or non-viral gene transduction system [including retrovirus (72), lentivirus (73), Sleeping Beauty transposon system (74) etc.]; (III) amplification in vitro [addition of cytokines for co-culture, such as IL2, IL15, IL7 and IL21 (75-77)]; (IV) reagent preparation and cryopreservation. After preparation, it is necessary for the researchers to formulate the appropriate injection scheme according to the patient's personal condition, such as injection method, dose, frequency, which reflects the characteristics of personalized treatment. However, due to lack of experience and standards, caution needs to be drawn up.
Compared with reports about the striking clinical effect of CAR-T cell therapy in blood cancer, it is pity that very few clinical studies of CAR-T cell therapy in liver disease were reported (only liver cancer) (56,76,77). In the case of HCC, for the first time, CD133.CAR-T cells have been used in treating 8 patients with advanced sorafenib refractory HCC (56). But some adverse events occurred in some patients during the treatment. It can be indicated that immunotherapy has achieved preferable results, but further studies are needed to avoid the generation of fatal side effects. In addition, during the process of hepatic arterial infusion (HAI) of CEA, CAR-T cells in 6 patients with LM of colon cancer, it was found that the neutrophil/lymphocyte ratio (NLR) and serum IL-6 levels were higher (78). At the same time, biopsy in 4 of the 6 patients showed an increase in liver metastatic necrosis or fibrosis, and none of these patients suffered from grade 3 or 4 adverse events associated with HAI of CAR-T cells, confirming the safety of CEA.CAR-T cells (78). In addition to the above clinical trials reported, there are still many clinical trials that are further testing the efficacy and safety of CAR-T cells, will providing more clinical data for us.
Comparative application of CAR-T in hematologic malignancies and liver cancer
Up to date, the enormous success of CAR-T cell therapy in hematologic malignancies has given researchers plenty of experience and lessons in conquering solid malignant tumors. Herein, we try to compare the different in CAR-T cell therapy between hematological malignancies and solid tumors represented by liver cancer from five aspects, hoping to give new inspirations to researchers.
TAA selection
Different TAAs are expressed on the surface of various tumor cells. It was firstly better goal for researchers to find ideal TAA. Previous studies have indicated that CD19 is a relatively ideal target in hematologic malignancies (79-86). On the one hand, CD19 was also expressed on the membrane of normal antigen presenting B cells, providing co-stimulatory receptors and ligands to enhance the activity of CAR-T cells (80). On the other hand, to some extent, CD19.CAR-T cells targeting normal B cells uncontrollably would lead to B cell aplasia (83). As B cells can be manually supplemented, compared with therapeutic effects, its toxicity can be negligible. Instead, TAAs in liver cancer tend to be expressed on the surface of some crucial tissues and cells that can not be replenished and replaced. If a suitable TAA for liver cancer will be found, it will greatly reduce the risk of treatment and improve the efficiency of anti-HCC. Besides, the scFv domain of CAR can be designed to target peptide-MHC complexes (87), to overcome the limitations for CAR-T cells targeting the only extracellular TAAs. For liver cancer, one representative example of intracellular TAAs targeted by CAR-T cells was AFP (53). Results demonstrated that AFP peptide-MHC complex CAR-T cells can effectively lysing AFP positive HCC in HepG2 tumor-bearing NOD/SCID IL-2 receptor gamma null (NSG) mouse model (53).
Pre-conditioning
For hematologic malignancies, in order to maintain the activity of CAR-T cells, usually lymphodepletion can be carried out before injection, such as high-dose chemotherapy or radiation, eliminating regulatory T cells (Tregs) and other immunosuppressor cells to diminish tumor burden and make enough room for infusion and proliferation of anti-tumor T lymphocyte (86,88). In the treatment of liver cancer, we can draw on the experience of preconditioning in hematologic malignancies to find the appropriate dose of chemotherapy.
Position difference
Because of the special physiological position of hematologic malignancies, when CAR-T cells enter the blood circulation intravenously, they can get in touch with hepatoma cells continuously, so as to obtain sustained activation and extend the persistence of CAR-T cells. However, different from the dispersion of hematologic malignancies, the position of liver cancer is fixed and single without such natural advantage.
Administration manner
In clinical, the adoptive transfer of autologous T cells was applicated for majority of patients. But, the method of adoptive transfer is diverse. Because of the physiological conditions in hematologic malignancies, adoptive transfer can be simply achieved by only intravenous injection (19). Unfortunately, the treatment of liver cancer is not as convenient as that. If administered intravenously, 49% of the CAR-T cells will remain in the lungs, and only the remaining enter the liver tissue via blood circulation (89,90). In addition, the peripheral matrix around the liver tissue automatically prevents a part of CAR-T cells into the core of the tumor, as a result significantly reducing the original efficacy. Thus, for solid tumors, intratumor/regional injection is beginning to develop, such as HAI for LM (78). In theory, the injection of regional CAR-T cells can compensate for poor T cell transport and increase targeting TAA, besides avoid systemic toxicity due to intravenous delivery, to significantly promote the efficacy of CAR-T cells.
IL-2 is the earliest confirmed cytokine that has been shown to play an important role in promoting the growth and amplification of T lymphocytes in vivo (91). A certain dose of IL-2 and CAR-T cells were synergetically administered at the same time in many clinical trials of solid tumors (78,92,93). However, IL-2 were not needed to added extra, because IL-2, IFN-γ, TNF-α and other cytokines were released by activated CAR-T cells during the treatment of hematologic malignancies (79).
Side effect
A variety of inflammatory cytokines were often released after CAR-T cells targeting tumor cells and being activated in vivo, resulting in fever, fatigue, muscle pain, nausea, anorexia, tachycardia, hypotension, capillary leakage, cardiac function incomplete, renal and liver failure, disseminated intravascular coagulation and so on clinical manifestations, which are collectively called cytokine release syndrome (CRS) (94). Interestingly, CRS often was occurred frequently during the treatment of patients with hematologic malignancies. However, CRS was rarely happened during the treatment of patients with HCC. Maybe, various factors in the procedure of treatment of liver cancer inhibit the activation of CAR-T cells, resulting in few cytokines being released (83,95).
The “on-target/off-tumor” effects are likely to occur in hematologic malignancies or liver cancer (83,96). Although CD19 is also expressed on surface of the normal B cells resulting in causing B cell aplasia in clinical applications, CD19 CAR-T cells are still attracted much attention in hematologic malignancies. In 2010, a high dose of third generation ERBB2 CAR-T cells were used to treat a woman with colon cancer metastatic to lung and liver. 15 minutes after infusion, the patient developed a respiratory distress and severe pulmonary infiltrate, and ultimately dead (96). It is probable that enormous administrated CAR-T cells in lung recognized low levels of ERBB2 on lung epithelial cells resulting in the cytokine releasing. It is an ultimate goal to get safety and effective CAR-T therapy for patients. So, more basic research and clinical trials are necessary.
Conclusions
Immunotherapy has broadened the new horizons of researchers to treat patients with advanced cancer. With the success of CD19.CAR-T cells in hematologic malignancies, the study of CAR-T cells is becoming more and more widespread in whole world. In 2017, a CAR-T cell therapy, Kymriah (TM) (CTL019) by Novartis, for B-cell acute lymphatic leukemia (ALL) was ratified by FDA (97). For liver diseases such as liver cancer, in pre-clinical experiments, it was found that CAR-T cells can lyse target cells highly efficiently. However, due to the complex human tumor microenvironment and other factors, the treatment of liver cancer with CAR-T cell therapy was not satisfied. Therefore, it is necessary to look for new TAA, modify the structure of CAR, improve the efficiency of injection, add adjuvant cytokines and perform combination therapy to improve CAR-T cells efficacy and safety in clinical trials. Meanwhile, more studies in other refractory solid tumors will also be assessed to develop CAR-T technology into a widespread cancer treatment strategy. Early trails in liver cancer have confirmed its feasibility, but the efficiency is limited and requires constant attempts and innovation. As a new approach to tumor immunotherapy, CAR-T cell therapy is expected to play a greater and more extensive role in the treatment of solid tumors in the future.
Acknowledgments
Funding: This work was supported by the Grants from the State Key Laboratory of Oncogenes and Related Genes (No. 90-15-04), the Natural Science Foundation of Jiangsu Province, China (BK20151347), Jiangsu Key Laboratory of Zoonosis (R1502).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/biotarget.2018.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duffy D, Mamdouh R, Laird M, et al. The ABCs of viral hepatitis that define biomarker signatures of acute viral hepatitis. Hepatology 2014;59:1273-82. [Crossref] [PubMed]
- Moirangthem A, Patel T. Mesenchymal stem cell derived extracellular vesicles: a promising new therapeutic approach for hepatic injury. Biotarget 2017;1:12. [Crossref]
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081-8. [Crossref] [PubMed]
- Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer 2013;13:123-35. [Crossref] [PubMed]
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720-4. [Crossref] [PubMed]
- Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 2012;14:405-15. [Crossref] [PubMed]
- Chakradhar. Driving CARs: As “living drugs”, T cell therapies face dose standardization woes. Nature Medicine 2015;221:1236-8.
- Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73 [Crossref] [PubMed]
- Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 2015;3:125-35. [Crossref] [PubMed]
- Park JH, Brentjens RJ. Are all chimeric antigen receptors created equal? J Clin Oncol 2015;33:651-3. [Crossref] [PubMed]
- Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 2002;20:70-5. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 2015;14:499-509. [Crossref] [PubMed]
- Hombach AA, Chmielewski M, Rappl G, et al. Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther 2013;24:259-69. [Crossref] [PubMed]
- Tang XY, Sun Y, Zhang A, et al. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 2016;6:e013904 [Crossref] [PubMed]
- Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 2015;15:1145-54. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia; Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia; Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med 2016;374:998. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell 1991;64:1037-46. [Crossref] [PubMed]
- Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res 2004;64:9160-6. [Crossref] [PubMed]
- Song DG, Ye Q, Santoro S, et al. Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther 2013;24:295-305. [Crossref] [PubMed]
- Lamers CH, Klaver Y, Gratama JW, et al. Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells-a completed study overview. Biochem Soc Trans 2016;44:951-9. [Crossref] [PubMed]
- Blat D, Zigmond E, Alteber Z, et al. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther 2014;22:1018-28. [Crossref] [PubMed]
- Li K, Lan Y, Wang J, et al. Chimeric antigen receptor-engineered T cells for liver cancers, progress and obstacles. Tumour Biol 2017;39:1010428317692229 [PubMed]
- Xu JY, Ye ZL, Jiang DQ, et al. Mesothelin-targeting chimeric antigen receptor-modified T cells by piggyBac transposon system suppress the growth of bile duct carcinoma. Tumour Biol 2017;39:1010428317695949 [Crossref] [PubMed]
- Song Y, Tong C, Wang Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Krebs K, Bottinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 2013;145:456-65. [Crossref] [PubMed]
- Bohne F, Chmielewski M, Ebert G, et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology 2008;134:239-47. [Crossref] [PubMed]
- Sautto GA, Wisskirchen K, Clementi N, et al. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut 2016;65:512-23. [Crossref] [PubMed]
- Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 2004;350:1118-29. [Crossref] [PubMed]
- Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006;44:675-84. [Crossref] [PubMed]
- Brimacombe CL, Grove J, Meredith LW, et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol 2011;85:596-605. [Crossref] [PubMed]
- Tarr AW, Lafaye P, Meredith L, et al. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology 2013;58:932-9. [Crossref] [PubMed]
- Sautto G, Tarr AW, Mancini N, et al. Structural and antigenic definition of hepatitis C virus E2 glycoprotein epitopes targeted by monoclonal antibodies. Clin Dev Immunol 2013;2013:450963.
- Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest 2001;108:497-501. [Crossref] [PubMed]
- Lei C, Long H, Li L, et al. Establishment of stable interference siRNA targeting GPC3 gene and its effects on the apoptosis of hepatocellular carcinoma cell line Huh-7. Chin J Clinicians 2014;8:3007-11.
- Dargel C, Bassani-Sternberg M, Hasreiter J, et al. T Cells Engineered to Express a T-Cell Receptor Specific for Glypican-3 to Recognize and Kill Hepatoma Cells In Vitro and in Mice. Gastroenterology 2015;149:1042-52. [Crossref] [PubMed]
- Baumhoer D, Tornillo L, Stadlmann S, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol 2008;129:899-906. [Crossref] [PubMed]
- Jiang Z, Jiang X, Chen S, et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front Immunol 2017;7:690. [PubMed]
- Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20:6418-28. [Crossref] [PubMed]
- Chen C, Li K, Jiang H, et al. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother 2017;66:475-89. [Crossref] [PubMed]
- Le Trinh T, Wu Q, Chang LJ, et al. GPC3-specific chimeric antigen receptor T cell in combination with Sorafenib as a novel therapeutic treatment for hepatocellular carcinoma. Cancer Research 2016;76:2316. [Crossref]
- Wu Y, Jiang M. The revolution of lung cancer treatment: from vaccines, to immune checkpoint inhibitors, to chimeric antigen receptor T therapy. Biotarget 2017;1:7. [Crossref]
- Wang J, Liu G, Li Q, et al. Mucin1 promotes the migration and invasion of hepatocellular carcinoma cells via JNK-mediated phosphorylation of Smad2 at the C-terminal and linker regions. Oncotarget 2015;6:19264-78. [PubMed]
- Li Q, Wang F, Liu G, et al. Impact of Mucin1 knockdown on the phenotypic characteristics of the human hepatocellular carcinoma cell line SMMC-7721. Oncol Rep 2014;31:2811-9. [Crossref] [PubMed]
- Ma YD, Wang Z, Gong R, et al. Specific cytotoxicity of MUC1 chimeric antigen receptor-engineered Jurkat T cells against hepatocellular carcinoma. Acad J Second Military Med Univ 2014;35:1177-82. [Crossref]
- Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis 2001;5:145-59. [Crossref] [PubMed]
- Bei R, Mizejewski GJ. Alpha fetoprotein is more than a hepatocellular cancer biomarker: from spontaneous immune response in cancer patients to the development of an AFP-based cancer vaccine. Curr Mol Med 2011;11:564-81. [Crossref] [PubMed]
- Smith JB. Alpha-fetoprotein: occurrence in certain malignant diseases and review of clinical applications. Med Clin North Am 1970;54:797-803. [PubMed]
- Liu H, Xu Y, Xiang J, et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin Cancer Res 2017;23:478-88. [Crossref] [PubMed]
- Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997;90:5002-12. [PubMed]
- Yin S, Li J, Hu C, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer 2007;120:1444-50. [Crossref] [PubMed]
- Sasaki A, Kamiyama T, Yokoo H, et al. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep 2010;24:537-46. [Crossref] [PubMed]
- Klapdor R, Wang S, Hacker U, et al. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum Gene Ther 2017;28:886-96. [Crossref] [PubMed]
- Zhu X, Prasad S, Gaedicke S, et al. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget 2015;6:171-84. [Crossref] [PubMed]
- Fichera A, Michelassi F, Arenas RB. Selective expression of carcinoembryonic antigen promoter in cancer cell lines: targeting strategy for gene therapy in colorectal cancer. Dis Colon Rectum 1998;41:747-54. [Crossref] [PubMed]
- Nap M, Mollgard K, Burtin P, et al. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol 1988;9:145-53. [Crossref] [PubMed]
- Yan Z, Deng X, Chen M, et al. Oncogenic c-Ki-ras but not oncogenic c-Ha-ras up-regulates CEA expression and disrupts basolateral polarity in colon epithelial cells. J Biol Chem 1997;272:27902-7. [Crossref] [PubMed]
- Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013;32:643-71. [Crossref] [PubMed]
- Donadon M, Ribero D, Morris-Stiff G, et al. New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res 2007;1:20-7. [PubMed]
- Emtage PC, Lo AS, Gomes EM, et al. Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res 2008;14:8112-22. [Crossref] [PubMed]
- Katz SC, Bamboat ZM, Maker AV, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 2013;20:946-55. [Crossref] [PubMed]
- Burga RA, Thorn M, Point GR, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 2015;64:817-29. [Crossref] [PubMed]
- Guo DL, Dong M, Wang L, et al. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol 2002;8:1009-13. [Crossref] [PubMed]
- Okazaki N, Seto T, Kanda Y, et al. Metastatic Pattern and Length of Survival in Patients With Liver Metastasis From Gastric Cancer. Jpn J Clin Oncol 1977;7:5-8. [Crossref]
- Vogl TJ, Gruber-Rouh T, Eichler K, et al. Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: local control and survival results. Eur J Radiol 2013;82:258-63. [Crossref] [PubMed]
- Makino H, Kunisaki C, Izumisawa Y, et al. Indication for hepatic resection in the treatment of liver metastasis from gastric cancer. Anticancer Res 2010;30:2367-76. [PubMed]
- Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86-91. [Crossref] [PubMed]
- Colovos C, Villena-Vargas J, Adusumilli PS. Safety and stability of retrovirally transduced chimeric antigen receptor T cells. Immunotherapy 2012;4:899-902. [Crossref] [PubMed]
- Kulemzin S, Chikaev N, Volkova O, et al. Modular lentiviral vector system for chimeric antigen receptor design optimization. Russ J Bioorgan Chem 2017;43:107-14. [Crossref]
- Maiti SN, Huls H, Singh H, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother 2013;36:112-23. [Crossref] [PubMed]
- Wilkie S, Burbridge SE, Chiapero-Stanke L, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem 2010;285:25538-44. [Crossref] [PubMed]
- Casucci M, Nicolis di Robilant B, Falcone L, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 2013;122:3461-72. [Crossref] [PubMed]
- Kaartinen T, Luostarinen A, Maliniemi P, et al. Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy 2017;19:1130. [Crossref] [PubMed]
- Katz SC, Burga RA, McCormack E, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res 2015;21:3149-59. [Crossref] [PubMed]
- An N, Tao Z, Li S, et al. Construction of a new anti-CD19 chimeric antigen receptor and the anti-leukemia function study of the transduced T cells. Oncotarget 2016;7:10638-49. [Crossref] [PubMed]
- Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817-28. [Crossref] [PubMed]
- Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 2016;7:12320. [Crossref] [PubMed]
- Jacoby E, Yang Y, Qin H, et al. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood 2016;127:1361-70. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709-20. [Crossref] [PubMed]
- Nellan A, Lee DW. Paving the road ahead for CD19 CAR T-cell therapy. Curr Opin Hematol 2015;22:516-20. [Crossref] [PubMed]
- Sabatino M, Hu J, Sommariva M, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood 2016;128:519-28. [Crossref] [PubMed]
- Zhang T, Cao L, Xie J, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget 2015;6:33961-71. [PubMed]
- Reiter Y, Di Carlo A, Fugger L, et al. Peptide-specific killing of antigen-presenting cells by a recombinant antibody-toxin fusion protein targeted to major histocompatibility complex/peptide class I complexes with T cell receptor-like specificity. Proc Natl Acad Sci U S A 1997;94:4631-6. [Crossref] [PubMed]
- Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907-12. [Crossref] [PubMed]
- Fisher B, Packard BS, Read EJ, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol 1989;7:250-61. [Crossref] [PubMed]
- Griffith KD, Read EJ, Carrasquillo JA, et al. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst 1989;81:1709-17. [Crossref] [PubMed]
- Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science 1984;224:1312-6. [Crossref] [PubMed]
- Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 2017;66:1425-36. [Crossref] [PubMed]
- Junghans RP, Ma Q, Rathore R, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016;76:1257-70. [Crossref] [PubMed]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. [Crossref] [PubMed]
- Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 2016;3:16006. [Crossref] [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [Crossref] [PubMed]
- Singh N, Shi J, June CH, et al. Genome-Editing Technologies in Adoptive T Cell Immunotherapy for Cancer. Curr Hematol Malig Rep 2017;12:522-9. [Crossref] [PubMed]
Cite this article as: Bai W, Wang P, Yu F. CAR-T cells shed light on the treatments of fatal liver diseases. Biotarget 2018;2:6.


