Overview of the neurokinin-1 receptor antagonists
Introduction
Neurokinin-1 (NK-1) receptor antagonists block substance P-mediated NK-1 receptors which are present in the abdominal vagus, the brainstem, and the area postrema in the brainstem. Substance P is a mammalian tachykinin that is found in vagal afferent neurons innervating the brainstem and sends impulses to the vomiting center (1). Compounds that block NK-1 receptors lessen emesis after cisplatin, ipecac, apomorphine, and radiation therapy (1).
Aprepitant and fosaprepitant
In 2003, the first NK-1 receptor antagonist, aprepitant (Emend, Merck), was approved for the control of chemotherapy-induced nausea and vomiting (CINV) when used in combination with a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist and dexamethasone (2,3). Aprepitant, an oral agent administered on the day of chemotherapy and daily for two days post-chemotherapy improved the control of acute and delayed CINV in combination with a 5-HT3 receptor antagonist and dexamethasone for patients receiving highly emetogenic chemotherapy (HEC) (2,3). There is little evidence that aprepitant is effective in the control of chemotherapy-induced nausea (2,4,5).
Fosaprepitant (also known as MK-0517 and L-758,298) is a water-soluble phosphoryl pro-drug for aprepitant which, when administered intravenously, is converted to aprepitant within 30 min via the action of phosphatase enzymes. Studies have demonstrated that a single dose of intravenous fosaprepitant, 150 mg on day 1 of cisplatin chemotherapy, was noninferior to a 3-day oral regimen of aprepitant in the prevention of CINV in the 120-h post-chemotherapy period (6).
Netupitant
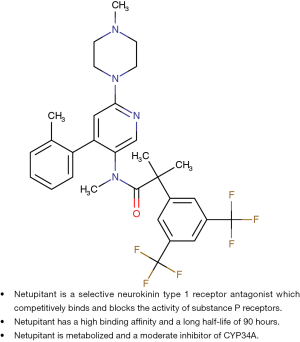
Netupitant is a new oral, potent, selective NK-1 receptor antagonist which is a potent brain penetrant agent for the targeting of NK-1 receptors according to positive emission tomography studies (7,8). Netupitant has a high degree of occupancy (90%) for a relatively long duration (96 h) when given as a single oral dose and appears to be well tolerated (8). Netupitant has a high binding affinity, and a relatively long half-life of 90 h compared to a 9 to 13 h half-life of aprepitant (3,7,8). It is metabolized by CYP3A4 and is a moderate inhibitor of CYP3A (3,7,8). Figure 1 illustrates the chemical structure and properties of netupitant compared to the structure and properties of aprepitant (3,7).
NEPA
NEPA is a combination of a fixed dose of netupitant (300 mg) and a fixed dose of palonosetron (0.50 mg) which was approved on October 10, 2014 (Akynzeo®, Helsinn Healthcare SA, Switzerland) by the US Food and Drug Administration (FDA) for the prevention of CINV in patients undergoing cancer chemotherapy (7,9-12). The approval was based on a number of phase II and III clinical trials for the prevention of CINV in patients receiving moderately emetogenic chemotherapy (MEC) and HEC (9-11).
A randomized, double-blind parallel group, dose-ranging study in 694 chemotherapy-naïve patients undergoing HEC (cisplatin-based chemotherapy) compared three different oral doses of netupitant (100, 200, and 300 mg) plus oral palonosetron (0.50 mg) (NEPA) with oral palonosetron (0.50 mg) with all agents given prior to chemotherapy (day 1). All patients in all treatment arms received oral dexamethasone on days 1 to 4 (9). All NEPA treatment arms of the study were significantly superior in overall complete response (CR) (no emesis, no use of rescue medications) rates compared to palonosetron alone. The 300 mg netupitant dose appeared to have a numerical advantage over the lower doses. There were no serious or differences in adverse events in any of treatment groups (9).
The 300 mg netupitant dose was employed in a randomized, double-blind, parallel group phase III study in 1,455 chemotherapy-naïve patients receiving MEC (including patients receiving anthracycline and cyclophosphamide). Patients were randomized to a single oral dose of NEPA (300 mg netupitant plus 0.50 mg palonosetron) or a single oral dose of palonosetron (0.50 mg) prior to chemotherapy, day 1. All patients received oral dexamethasone on day 1 only. The CR, no emesis and no use of rescue medications, during the delayed period was significantly higher for the NEPA group of patients compared to the palonosetron patient group. The patients who received NEPA had a similar safety profile as the patients who received palonosetron (10).
The patients receiving NEPA in the HEC study (9) and the MEC study (10) were further evaluated to determine the safety and efficacy of NEPA over multiple cycles of chemotherapy (11). In the multi-cycle patient group, 75% completed at least four cycles, and 40% completed six cycles. The CR rates were maintained over repeated cycles for the NEPA patient group. The adverse events were mild/moderate with no cardiac safety concerns (11).
In an attempt to explore the degree of nausea control with the use of NEPA compared to palonosetron, patients from two randomized, multinational studies (9,10) who received a single dose of NEPA (netupitant 300 mg plus palonosetron 0.50 mg) or palonosetron and dexamethasone prior to cisplatin or an anthracycline plus cyclophosphamide were evaluated for no significant nausea (≤25, 0–100 mm, visual analog scale). The NEPA group had more patients with no significant nausea; this appeared to be most apparent in the delayed nausea phase of the patients receiving cisplatin (12).
Rolapitant
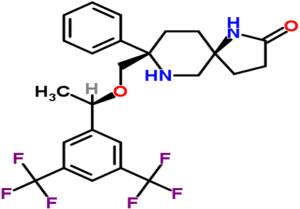
Rolapitant is a high affinity, highly-selective NK-1 receptor antagonist (13). It penetrates the central nervous system following oral administration, and it has a high affinity for the human NK-1 receptor and is highly selective over the human NK-2 and NK-3 receptor subtypes. It is a functionally competitive antagonist and reversed NK-1 agonist-induced foot tapping in a gerbil animal model following both intravenous and oral intravenous and oral administration (13). Rolapitant reverses both apomorphine and cisplatin-induced emesis in ferrets (13). Figure 2 illustrates the chemical structure of rolapitant.
The pharmacokinetics of rolapitant demonstrates that it has a long half-life (approximately 180 h) with high affinity (Ki =0.66 nM) for the NK-1 receptor (13,14), and it does not induce or inhibit CYP3A4. Poma et al. (14) reported that rolapitant and its major metabolite SCH720 881 do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Rolapitant does not induce CYP3A4, and single oral doses of rolapitant, co-administered with midazolam were safe and well tolerated. Administration of rolapitant, unlike other NK-1 receptor antagonists aprepitant and netupitant, does not require dose adjustment of concomitantly administered drugs metabolized by CYP34A.
The pharmacodynamic data of rolapitant showed that a 180 mg rolapitant dose provided ≥90% NK-1 receptor occupancy in the brain up to 5 days following a single dose (15).
A phase II randomized, double-blind, active-controlled study was performed to evaluate the safety and efficacy of four different doses of rolapitant for the prevention of CINV in patients receiving HEC (16). Patients receiving cisplatin-based chemotherapy (≥70 mg/m2) received 9, 22.5, 90, or 180 mg of oral rolapitant or placebo in addition to ondansetron and dexamethasone on day 1 of chemotherapy. Four hundred and fifty-four patients were randomized. All doses of rolapitant improved CR compared to placebo with the 180 mg rolapitant dose demonstrating the greatest benefit. The prevention of emesis with the 180 mg dose of rolapitant was significantly improved compared to placebo in the acute, delayed, and overall periods. Adverse events were mild or moderate and similar across treatment group.
In a randomized, double-blind, active-controlled, parallel-group, phase III study, patients receiving MEC received 180 mg of oral rolapitant or placebo in addition to granisetron and dexamethasone on day 1 prior to chemotherapy as well as granisetron on days 2 and 3 post- chemotherapy. One thousand and forty-four patients were randomized with approximately 50% of the patients receiving anthracycline/cyclophosphamide chemotherapy. The CR rate was significantly higher in the delayed phase (>24–120 h) for patients receiving rolapitant. The incidence of adverse events was similar in the rolapitant and control groups, with the most commonly reported treatment-related treatment-emergent adverse events being fatigue, constipation, and headache. No nausea (0, 0–100 mm, visual analogue scale) and no significant nausea, secondary endpoints, were not improved with rolapitant. Rolapitant in combination with granisetron and dexamethasone was well tolerated and demonstrated superiority over active control for the prevention of delayed emesis in patients receiving MEC (17).
In two global, randomized, double-blind, active-controlled, parallel-group, phase III studies, patients receiving cisplatin-based HEC received an oral 180 mg rolapitant dose or placebo in addition to granisetron and dexamethasone on day 1 prior to chemotherapy as well as 8 mg of oral dexamethasone on days 2–4 post-chemotherapy before HEC administration. Five-hundred and twenty-six patients were randomized in study 1 and 544 patients were randomized in study 2. The primary endpoint, CR rate in the delayed phase (>24–120 h) was significantly higher for the rolapitant-treated patients compared to the control group in both study 1 and study 2. No nausea and no significant nausea, secondary endpoints, were significantly (P<0.05) improved in the delayed and overall periods with rolapitant in one [HEC-1 of the two (HEC-1, HEC-2] studies (18). The incidence of adverse events was similar across treatment groups. The most commonly reported treatment-related events were headache, hiccups, constipation, and dyspepsia. Rolapitant in combination with granisetron and dexamethasone was well-tolerated and superior over active control for the prevention of prevention of delayed emesis in patients receiving HEC (18).
Based on the phase II and phase III clinical trials, in September, 2015, the US FDA approved rolapitant in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, HEC.
Comparison of NK-1 receptor antagonists
At present, there are no definitive published clinical trials reporting a comparison of the efficacy and safety of the various NK-1 receptor antagonists (aprepitant, fosaprepitant, netupitant, rolapitant). One of the NEPA clinical trials involving patients receiving HEC included a comparative arm consisting of oral aprepitant plus intravenous ondansetron. All patients in all arms received standard doses of dexamethasone. Based on the data reported in the NEPA clinical trial (9), there appeared to be no significant differences in the prevention of CINV between NEPA and the aprepitant and ondansetron combination (7,9). A formal statistical comparison of the NEPA and aprepitant/ondansetron arms was not reported (9).
At the 2017 ASCO international annual meeting, Zhang et al. is scheduled to report a large phase III study of 828 patients who were randomized to receive NEPA plus dexamethasone or oral aprepitant, granisetron, and dexamethasone for the prevention of CINV in patients receiving cisplatin based HEC. The study abstract reports that NEPA was non-inferior to aprepitant/granisetron in overall CR (19).
Zhang et al. recently reported a meta-analysis (36 trials, 18,889 patients) of the NK-1 receptor antagonist-based triple regimens (NK-1, 5-HT3 receptor antagonists, dexamethasone) in preventing CINV. The analysis reported that the triple regimens involving any of the available NK-1 receptor antagonists (aprepitant, netupitant, rolapitant) were significantly better in controlling CINV in the acute, delayed, and overall periods in patient receiving HEC compared to duplex (5-HT3, dexamethasone) antiemetic regimens (20). The analysis also reported that only aprepitant-based triple regimens demonstrated an improvement in CINV control in patients receiving MEC. In the analysis, palonosetron-based triple regimens were reported to share equivalent effect on CINV control with the first generation 5-HT3 receptor antagonists-based triple regimens and different doses of dexamethasone plus NK-1 receptor antagonists and 5-HT3 receptor antagonists were reported to show no statistically significant difference in CR.
The finding in the meta-analysis that the addition of any the various available NK-1s to a 5-HT3 receptor antagonist and dexamethasone improved the control of CINV in patients receiving HEC compared to a 5-HT3 receptor antagonist and dexamethasone alone is an expected result based on the available studies in the literature. All of the international antiemetic guidelines (NCCN, ASCO, MASCC/ESMO) recommend the use of the three drug regimen (any of the available NK-1s, a 5-HT3, and dexamethasone) for the control of CINV in patients receiving HEC (21-24).
The finding in the meta-analysis that only aprepitant-based triple regimens demonstrated an improvement in CINV control in patients receiving MEC is curious in that the FDA registration studies for both NEPA (10) and rolapitant (17) demonstrated an improvement in the control of CINV when NEPA or Rolapitant was used in a triplet antiemetic regimen in patients receiving MEC. The international antiemetic guidelines (21-24) differ on the recommendation of the benefit of adding an NK-1 receptor antagonist to a 5-HT3 receptor antagonist and dexamethasone for patients receiving MEC. The guidelines suggest that not all MEC regimens may require the addition of an NK-1 receptor antagonist to a 5-HT3 receptor antagonist and dexamethasone for adequate control of CINV (21-24), and the specific MEC regimen to be received be considered to determine if an NK-1 receptor antagonists is necessary. The NCCN guidelines (23) suggest a triplet antiemetic regimen for carboplatin and oxaliplatin; the MASCC/ESMO guidelines (22) recommend a triplet antiemetic regimen for carboplatin chemotherapy.
The suggestions by the meta-analysis that palonosetron-based triple regimens shared equivalent effect on CINV control with the first generation 5-HT3 receptor antagonists-based triple regimens and that different doses of dexamethasone plus NK-1 receptor antagonists and 5-HT3 receptor antagonists showed no statistically significant difference in CR are most likely not justified. There are few, if any, randomized double-blind phase III studies which compared palonosetron to a first generation 5-HT3 receptor antagonist using the same NK-1 receptor antagonist and dexamethasone. Similarly, there are few studies comparing different doses of dexamethasone in a triplet antiemetic regimen.
The meta-analysis focused on the effect of the triplet antiemetic regimens on the end point of CR, no emesis and no use of rescue medications, but did not report on the control of nausea. Based on the available clinical trial data, the NK-1 receptor antagonists have significantly improved the prevention of acute and delayed emesis in patients receiving moderately or HEC, but there is little evidence that these agents are effective in controlling nausea (2,4,5,7). Recent reviews have concluded that aprepitant has little effect on the prevention of chemotherapy-induced nausea (2,4,5). In a subgroup analysis of patients receiving cisplatin or an anthracycline plus cyclophosphamide, data from two clinical trials demonstrated that NEPA may have improved no significant nausea (a secondary endpoint) compared to palonosetron (12). The rolapitant clinical trials showed no improvement in the control of nausea in the patients receiving MEC (17) and improvement in the control of nausea in one of the two trials in patients receiving HEC (18).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Zhang Yaxiong (Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China; State Key laboratory of Oncology in South China, Guangzhou, China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/biotarget.2017.06.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Diemunsch P, Grélot L. Potential of substance P antagonists as antiemetics. Drugs 2000;60:533-46. [Crossref] [PubMed]
- Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 2013;73:249-62. [Crossref] [PubMed]
- Aapro M, Carides A, Rapoport BL, et al. Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist 2015;20:450-8. [Crossref] [PubMed]
- Ng TL, Hutton B, Clemons M, et al. Chemotherapy-Induced Nausea and Vomiting: Time for More Emphasis on Nausea? Oncologist 2015;20:576-83. [Crossref] [PubMed]
- Navari RM. Treatment of chemotherapy-induced nausea. Community Oncol 2012;9:20-6. [Crossref] [PubMed]
- Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol--EASE. J Clin Oncol 2011;29:1495-501. [Crossref] [PubMed]
- Navari RM. Profile of netupitant/palonosetron (NEPA) fixed dose combination and its potential in the treatment of chemotherapy-induced nausea and vomiting (CINV). Drug Des Devel Ther 2014;9:155-61. [Crossref] [PubMed]
- Spinelli T, Calcagneli S, Giuliano C, et al. Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol 2014;54:97-108. [Crossref] [PubMed]
- Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 2014;25:1340-6. [Crossref] [PubMed]
- Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328-33. [Crossref] [PubMed]
- Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 2014;25:1333-9. [Crossref] [PubMed]
- Schwartzberg L, Aapro M, Hesketh PJ, et al. Do NK1 receptor antagonists (RA) contribute to nausea control? Evaluation of the novel NEPA fixed-dose combination of NK1 RA + 5-HT3 RA from pivotal trials. Support Care Cancer 2014;22:107. Abstract 161.
- Duffy RA, Morgan C, Naylor R, et al. Rolapitant (SCH 619734): a potent, selective and orally active neurokinin NK1 receptor antagonist with centrally-mediated antiemetic effects in ferrets. Pharmacol Biochem Behav 2012;102:95-100. [Crossref] [PubMed]
- Poma A, Christensen J, Pertikis H, et al. Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Support Care Cancer 2013;21:S154. Abstract 441.
- Poma A, Christensen J, Davis J, et al. Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol 2014;32: abstr e20690.
- Rapoport B, Chua D, Poma A, et al. Study of rolapitant, a novel, long-acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer 2015;23:3281-8. [Crossref] [PubMed]
- Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol 2015;16:1071-8. [Crossref] [PubMed]
- Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol 2015;16:1079-89. [Crossref] [PubMed]
- Zhang L, Lu S, Feng F, et al. Phase III study of NEPA, a fixed combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV). J Clin Oncol 2017;35: (suppl; abstr 10090).
- Zhang Y, Yang Y, Zhang Z, et al. Neurokinin-1 Receptor Antagonist-Based Triple Regimens in Preventing Chemotherapy-Induced Nausea and Vomiting: A Network Meta-Analysis. J Natl Cancer Inst 2016;109: [PubMed]
- Herrstedt J, Roila F, Warr D, et al. 2016 Updated MASCC/ESMO Consensus Recommendations: Prevention of Nausea and Vomiting Following High Emetic Risk Chemotherapy. Support Care Cancer 2017;25:277-88. [Crossref] [PubMed]
- Roila F, Warr D, Hesketh PJ, et al. 2016 updated MASCC/ESMO consensus recommendations: Prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer 2017;25:289-94. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology version 2.2017. Anti¬emesis. National Comprehensive Cancer Network (NCCN). 2017. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf
- Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology Focused Guideline Update. J Clin Oncol 2016;34:381-6. [Crossref] [PubMed]
Cite this article as: Navari RM. Overview of the neurokinin-1 receptor antagonists. Biotarget 2017;:8.